Nature ( IF 64.8 ) Pub Date : 2020-01-15 , DOI: 10.1038/s41586-019-1915-7 Lin Wang 1 , Juehui Wu 1, 2 , Jun Li 3 , Hua Yang 1 , Tianqi Tang 2 , Haijiao Liang 1, 2 , Mianyong Zuo 2 , Jie Wang 1 , Haipeng Liu 1, 4 , Feng Liu 1 , Jianxia Chen 1, 4 , Zhonghua Liu 1 , Yang Wang 2 , Cheng Peng 2 , Xiangyang Wu 2 , Ruijuan Zheng 1 , Xiaochen Huang 1 , Yajun Ran 3 , Zihe Rao 3, 5, 6, 7 , Baoxue Ge 1, 2, 4
|
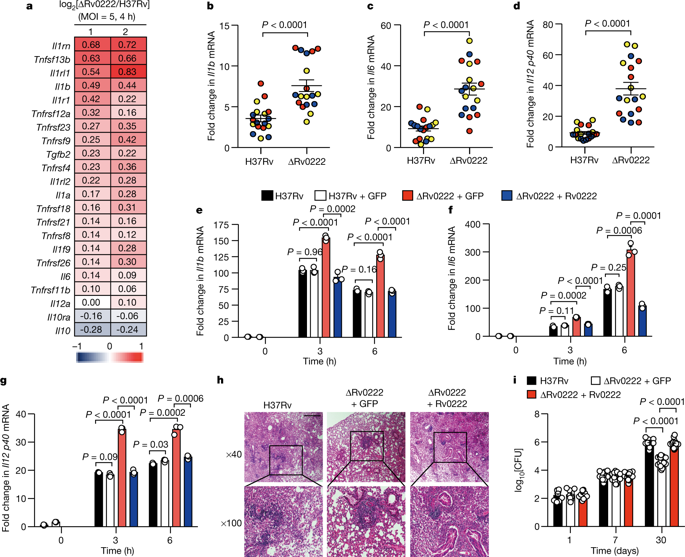
Mycobacterium tuberculosis is an intracellular pathogen that uses several strategies to interfere with the signalling functions of host immune molecules. Many other bacterial pathogens exploit the host ubiquitination system to promote pathogenesis1,2, but whether this same system modulates the ubiquitination of M. tuberculosis proteins is unknown. Here we report that the host E3 ubiquitin ligase ANAPC2—a core subunit of the anaphase-promoting complex/cyclosome—interacts with the mycobacterial protein Rv0222 and promotes the attachment of lysine-11-linked ubiquitin chains to lysine 76 of Rv0222 in order to suppress the expression of proinflammatory cytokines. Inhibition of ANAPC2 by specific short hairpin RNA abolishes the inhibitory effect of Rv0222 on proinflammatory responses. Moreover, mutation of the ubiquitination site on Rv0222 impairs the inhibition of proinflammatory cytokines by Rv0222 and reduces virulence during infection in mice. Mechanistically, lysine-11-linked ubiquitination of Rv0222 by ANAPC2 facilitates the recruitment of the protein tyrosine phosphatase SHP1 to the adaptor protein TRAF6, preventing the lysine-63-linked ubiquitination and activation of TRAF6. Our findings identify a previously unrecognized mechanism that M. tuberculosis uses to suppress host immunity, and provide insights relevant to the development of effective immunomodulators that target M. tuberculosis.
中文翻译:

宿主介导的分枝杆菌蛋白泛素化抑制免疫
结核分枝杆菌是一种细胞内病原体,它使用多种策略来干扰宿主免疫分子的信号传导功能。许多其他细菌病原体利用宿主泛素化系统来促进发病机制1,2,但该系统是否会调节M. tuberculosis的泛素化蛋白质未知。在这里,我们报告宿主 E3 泛素连接酶 ANAPC2(后期促进复合物/环体的核心亚基)与分枝杆菌蛋白 Rv0222 相互作用并促进赖氨酸 11 连接的泛素链与 Rv0222 的赖氨酸 76 的附着,以抑制促炎细胞因子的表达。特异性短发夹 RNA 对 ANAPC2 的抑制消除了 Rv0222 对促炎反应的抑制作用。此外,Rv0222 上泛素化位点的突变会削弱 Rv0222 对促炎细胞因子的抑制作用,并降低小鼠感染期间的毒力。机制上,ANAPC2 对 Rv0222 的赖氨酸 11 连接泛素化促进了蛋白酪氨酸磷酸酶 SHP1 向衔接蛋白 TRAF6 的募集,阻止赖氨酸63连接的泛素化和TRAF6的激活。我们的研究结果确定了一种以前未被认识的机制,结核分枝杆菌用于抑制宿主免疫,并提供与开发针对结核分枝杆菌的有效免疫调节剂相关的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号