当前位置:
X-MOL 学术
›
Curr. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Cycle of Ubiquitination Regulates Adaptor Function of the Nedd4-Family Ubiquitin Ligase Rsp5.
Current Biology ( IF 9.2 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.cub.2019.11.086 Chris MacDonald 1 , S Brookhart Shields 1 , Charlotte A Williams 1 , Stanley Winistorfer 1 , Robert C Piper 1
Current Biology ( IF 9.2 ) Pub Date : 2020-01-16 , DOI: 10.1016/j.cub.2019.11.086 Chris MacDonald 1 , S Brookhart Shields 1 , Charlotte A Williams 1 , Stanley Winistorfer 1 , Robert C Piper 1
Affiliation
|
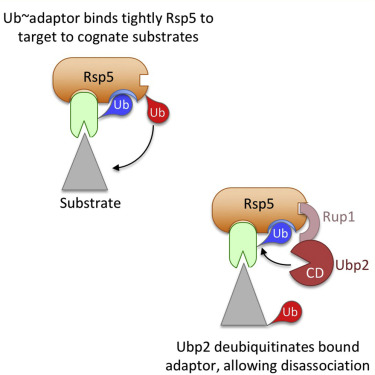
In yeast, the main ubiquitin ligase responsible for the sorting of proteins to the lysosomal vacuole is Rsp5, a member of the Nedd4 family of ligases whose distinguishing features are a catalytic homologous to E6AP C terminus (HECT) domain and 3 central WW domains that bind PY motifs in target proteins. Many substrates do not bind Rsp5 directly and instead rely on PY-containing adaptor proteins that interact with Rsp5. Recent studies indicate that the activities of these adaptors are elevated when they undergo ubiquitination, yet the mechanism whereby ubiquitination activates the adaptors and how this process is regulated remain unclear. Here, we report on a mechanism that explains how ubiquitination stimulates adaptor function and how this process can be regulated by the Rsp5-associated deubiquitinase, Ubp2. Our overexpression experiments revealed that several adaptors compete for Rsp5 in vivo. We found that the ability of the adaptors to compete effectively was enhanced by their ubiquitination and diminished by a block of their ubiquitination. Ubiquitination-dependent adaptor activation required a ubiquitin-binding surface within the Rsp5 catalytic HECT domain. Finally, like constitutively ubiquitinated adaptors, a Ubp2 deficiency increased both the adaptor activity and the ability to compete for Rsp5. Our data support a model whereby ubiquitinated Rsp5 adaptors are more active when "locked" onto Rsp5 via its N-lobe ubiquitin-binding surface and less active when they are "unlocked" by Ubp2-mediated deubiquitination.
中文翻译:

泛素化循环调节 Nedd4 家族泛素连接酶 Rsp5 的衔接功能。
在酵母中,负责将蛋白质分类到溶酶体液泡的主要泛素连接酶是 Rsp5,它是连接酶 Nedd4 家族的成员,其显着特征是与 E6AP C 末端 (HECT) 结构域和结合的 3 个中心 WW 结构域同源的催化同源物目标蛋白中的 PY 基序。许多底物不直接结合 Rsp5,而是依赖于与 Rsp5 相互作用的含 PY 的衔接蛋白。最近的研究表明,当这些衔接子进行泛素化时,它们的活性会升高,但泛素化激活衔接子的机制以及该过程的调控方式仍不清楚。在这里,我们报告了一种机制,该机制解释了泛素化如何刺激适配器功能以及该过程如何受 Rsp5 相关去泛素化酶 Ubp2 的调节。我们的过表达实验表明,几种衔接子在体内竞争 Rsp5。我们发现,适配器有效竞争的能力因它们的泛素化而增强,并因它们的泛素化阻滞而减弱。泛素化依赖性适配器激活需要 Rsp5 催化 HECT 域内的泛素结合表面。最后,与组成型泛素化适配器一样,Ubp2 缺陷增加了适配器活动和竞争 Rsp5 的能力。我们的数据支持一种模型,其中泛素化 Rsp5 适配器在通过其 N 叶泛素结合表面“锁定”到 Rsp5 时更加活跃,而在被 Ubp2 介导的去泛素化“解锁”时则不那么活跃。我们发现,适配器有效竞争的能力因它们的泛素化而增强,并因它们的泛素化阻滞而减弱。泛素化依赖性适配器激活需要 Rsp5 催化 HECT 域内的泛素结合表面。最后,与组成型泛素化适配器一样,Ubp2 缺陷增加了适配器活动和竞争 Rsp5 的能力。我们的数据支持一种模型,其中泛素化 Rsp5 适配器在通过其 N 叶泛素结合表面“锁定”到 Rsp5 时更加活跃,而在被 Ubp2 介导的去泛素化“解锁”时则不那么活跃。我们发现,适配器有效竞争的能力因它们的泛素化而增强,并因它们的泛素化阻滞而减弱。泛素化依赖性适配器激活需要 Rsp5 催化 HECT 域内的泛素结合表面。最后,与组成型泛素化适配器一样,Ubp2 缺陷增加了适配器活动和竞争 Rsp5 的能力。我们的数据支持一种模型,其中泛素化 Rsp5 适配器在通过其 N 叶泛素结合表面“锁定”到 Rsp5 时更加活跃,而在被 Ubp2 介导的去泛素化“解锁”时则不那么活跃。最后,与组成型泛素化适配器一样,Ubp2 缺陷增加了适配器活动和竞争 Rsp5 的能力。我们的数据支持一种模型,其中泛素化 Rsp5 适配器在通过其 N 叶泛素结合表面“锁定”到 Rsp5 时更加活跃,而在被 Ubp2 介导的去泛素化“解锁”时则不那么活跃。最后,与组成型泛素化适配器一样,Ubp2 缺陷增加了适配器活动和竞争 Rsp5 的能力。我们的数据支持一种模型,其中泛素化 Rsp5 适配器在通过其 N 叶泛素结合表面“锁定”到 Rsp5 时更加活跃,而在被 Ubp2 介导的去泛素化“解锁”时则不那么活跃。
更新日期:2020-01-16
中文翻译:

泛素化循环调节 Nedd4 家族泛素连接酶 Rsp5 的衔接功能。
在酵母中,负责将蛋白质分类到溶酶体液泡的主要泛素连接酶是 Rsp5,它是连接酶 Nedd4 家族的成员,其显着特征是与 E6AP C 末端 (HECT) 结构域和结合的 3 个中心 WW 结构域同源的催化同源物目标蛋白中的 PY 基序。许多底物不直接结合 Rsp5,而是依赖于与 Rsp5 相互作用的含 PY 的衔接蛋白。最近的研究表明,当这些衔接子进行泛素化时,它们的活性会升高,但泛素化激活衔接子的机制以及该过程的调控方式仍不清楚。在这里,我们报告了一种机制,该机制解释了泛素化如何刺激适配器功能以及该过程如何受 Rsp5 相关去泛素化酶 Ubp2 的调节。我们的过表达实验表明,几种衔接子在体内竞争 Rsp5。我们发现,适配器有效竞争的能力因它们的泛素化而增强,并因它们的泛素化阻滞而减弱。泛素化依赖性适配器激活需要 Rsp5 催化 HECT 域内的泛素结合表面。最后,与组成型泛素化适配器一样,Ubp2 缺陷增加了适配器活动和竞争 Rsp5 的能力。我们的数据支持一种模型,其中泛素化 Rsp5 适配器在通过其 N 叶泛素结合表面“锁定”到 Rsp5 时更加活跃,而在被 Ubp2 介导的去泛素化“解锁”时则不那么活跃。我们发现,适配器有效竞争的能力因它们的泛素化而增强,并因它们的泛素化阻滞而减弱。泛素化依赖性适配器激活需要 Rsp5 催化 HECT 域内的泛素结合表面。最后,与组成型泛素化适配器一样,Ubp2 缺陷增加了适配器活动和竞争 Rsp5 的能力。我们的数据支持一种模型,其中泛素化 Rsp5 适配器在通过其 N 叶泛素结合表面“锁定”到 Rsp5 时更加活跃,而在被 Ubp2 介导的去泛素化“解锁”时则不那么活跃。我们发现,适配器有效竞争的能力因它们的泛素化而增强,并因它们的泛素化阻滞而减弱。泛素化依赖性适配器激活需要 Rsp5 催化 HECT 域内的泛素结合表面。最后,与组成型泛素化适配器一样,Ubp2 缺陷增加了适配器活动和竞争 Rsp5 的能力。我们的数据支持一种模型,其中泛素化 Rsp5 适配器在通过其 N 叶泛素结合表面“锁定”到 Rsp5 时更加活跃,而在被 Ubp2 介导的去泛素化“解锁”时则不那么活跃。最后,与组成型泛素化适配器一样,Ubp2 缺陷增加了适配器活动和竞争 Rsp5 的能力。我们的数据支持一种模型,其中泛素化 Rsp5 适配器在通过其 N 叶泛素结合表面“锁定”到 Rsp5 时更加活跃,而在被 Ubp2 介导的去泛素化“解锁”时则不那么活跃。最后,与组成型泛素化适配器一样,Ubp2 缺陷增加了适配器活动和竞争 Rsp5 的能力。我们的数据支持一种模型,其中泛素化 Rsp5 适配器在通过其 N 叶泛素结合表面“锁定”到 Rsp5 时更加活跃,而在被 Ubp2 介导的去泛素化“解锁”时则不那么活跃。


























 京公网安备 11010802027423号
京公网安备 11010802027423号