当前位置:
X-MOL 学术
›
Mol. Ther. Methods Clin. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Digital PCR Assays for Precise Quantification of CD19-CAR-T Cells after Treatment with Axicabtagene Ciloleucel.
Molecular Therapy - Methods & Clinical Development ( IF 4.7 ) Pub Date : 2020-01-15 , DOI: 10.1016/j.omtm.2019.12.018 Boris Fehse 1, 2 , Anita Badbaran 1 , Carolina Berger 1 , Tanja Sonntag 1, 2 , Kristoffer Riecken 1, 2 , Maria Geffken 3 , Nicolaus Kröger 1 , Francis A Ayuk 1
Molecular Therapy - Methods & Clinical Development ( IF 4.7 ) Pub Date : 2020-01-15 , DOI: 10.1016/j.omtm.2019.12.018 Boris Fehse 1, 2 , Anita Badbaran 1 , Carolina Berger 1 , Tanja Sonntag 1, 2 , Kristoffer Riecken 1, 2 , Maria Geffken 3 , Nicolaus Kröger 1 , Francis A Ayuk 1
Affiliation
|
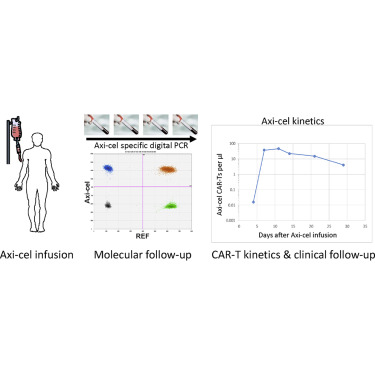
Treatment with axicabtagene ciloleucel (Axi-cel) CD19-CAR-T (chimeric antigen receptor T) cells has been approved for refractory/relapsed diffuse large B cell lymphoma (DLBCL) and primary mediastinal large B cell lymphoma (PMBCL). Because treatment success as well as side effects might depend on CAR-T cell expansion in vivo, we aimed at developing digital PCR (dPCR) assays for detection and quantification of CAR-T cells. To this end, we cloned and sequenced the complete cDNA of the CAR construct. We designed different combinations of primers and dual-labeled hydrolysis probes located in various CAR regions. Three combinations were successfully tested on CAR-positive and -negative cells in duplex reactions with a reference gene (REF) to concomitantly assess cell numbers. All assays demonstrated excellent specificity and reproducibility with neglectable inter-assay variations. For all three assays, almost perfect correlation between the two dPCRs (Axi-cel versus REF) was observed, and the limit of detection was one single CAR-transduced cell corresponding to a sensitivity of 0.01% for 100 ng genomic DNA. After cross-validation, we used one assay to monitor Axi-cel CAR-T numbers in patients. CAR-T expansion and contraction followed the expected kinetics with median peak value of 11.2 Axi-cel CAR-T cells/μL at 11.3 days (median). Clinically, we observed only two partial responses (PRs) in the five patients with CAR-T cell peak numbers below median, whereas four of the five patients with comparatively good expansion showed clinical responses (two complete responses [CRs] and two PRs) on day 30. In conclusion, we established a novel dPCR assay for the sensitive detection of transgenic CAR-T cells, which should be very useful in the context of Axi-cel treatment.
中文翻译:

用Axicabtagene Ciloleucel处理后,精确定量CD19-CAR-T细胞的数字PCR分析。
已批准使用轴抗角蛋白(Axi-cel)CD19-CAR-T(嵌合抗原受体T)细胞治疗难治性/复发性弥漫性大B细胞淋巴瘤(DLBCL)和原发性纵隔大B细胞淋巴瘤(PMBCL)。由于治疗的成功与副作用可能取决于体内CAR-T细胞的扩增,因此我们旨在开发用于检测和定量CAR-T细胞的数字PCR(dPCR)分析。为此,我们克隆并测序了CAR构建体的完整cDNA。我们设计了位于不同CAR区域的引物和双标记水解探针的不同组合。与参考基因(REF)进行双重反应后,成功在CAR阳性和阴性细胞上测试了三种组合,以同时评估细胞数。所有测定法均显示出极好的特异性和可重复性,而测定法间的差异可忽略不计。对于所有三种测定,观察到两个dPCR之间的几乎完美的相关性(Axi-cel与REF),检出限为一个单个CAR转导的细胞,对100 ng基因组DNA的敏感性为0.01%。交叉验证后,我们使用了一种测定法来监测患者的Axi-cel CAR-T数量。CAR-T的扩张和收缩遵循预期的动力学,在11.3天(中位数)的中位峰值为11.2 Axi-cel CAR-T细胞/μL。在临床上,我们观察到5例CAR-T细胞峰值低于中位值的患者中只有2个局部反应(PR),而5例扩张性相对较好的患者中有4例表现出临床反应(2个完全反应[CR]和2个PRs)。第30天。总而言之,
更新日期:2020-01-15
中文翻译:

用Axicabtagene Ciloleucel处理后,精确定量CD19-CAR-T细胞的数字PCR分析。
已批准使用轴抗角蛋白(Axi-cel)CD19-CAR-T(嵌合抗原受体T)细胞治疗难治性/复发性弥漫性大B细胞淋巴瘤(DLBCL)和原发性纵隔大B细胞淋巴瘤(PMBCL)。由于治疗的成功与副作用可能取决于体内CAR-T细胞的扩增,因此我们旨在开发用于检测和定量CAR-T细胞的数字PCR(dPCR)分析。为此,我们克隆并测序了CAR构建体的完整cDNA。我们设计了位于不同CAR区域的引物和双标记水解探针的不同组合。与参考基因(REF)进行双重反应后,成功在CAR阳性和阴性细胞上测试了三种组合,以同时评估细胞数。所有测定法均显示出极好的特异性和可重复性,而测定法间的差异可忽略不计。对于所有三种测定,观察到两个dPCR之间的几乎完美的相关性(Axi-cel与REF),检出限为一个单个CAR转导的细胞,对100 ng基因组DNA的敏感性为0.01%。交叉验证后,我们使用了一种测定法来监测患者的Axi-cel CAR-T数量。CAR-T的扩张和收缩遵循预期的动力学,在11.3天(中位数)的中位峰值为11.2 Axi-cel CAR-T细胞/μL。在临床上,我们观察到5例CAR-T细胞峰值低于中位值的患者中只有2个局部反应(PR),而5例扩张性相对较好的患者中有4例表现出临床反应(2个完全反应[CR]和2个PRs)。第30天。总而言之,



























 京公网安备 11010802027423号
京公网安备 11010802027423号