当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Site-Selective C-H Functionalization via Synergistic Use of Electrochemistry and Transition Metal Catalysis.
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-01-15 , DOI: 10.1021/acs.accounts.9b00603 Ke-Jin Jiao 1 , Yi-Kang Xing 1 , Qi-Liang Yang 1 , Hui Qiu 1 , Tian-Sheng Mei 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-01-15 , DOI: 10.1021/acs.accounts.9b00603 Ke-Jin Jiao 1 , Yi-Kang Xing 1 , Qi-Liang Yang 1 , Hui Qiu 1 , Tian-Sheng Mei 1
Affiliation
|
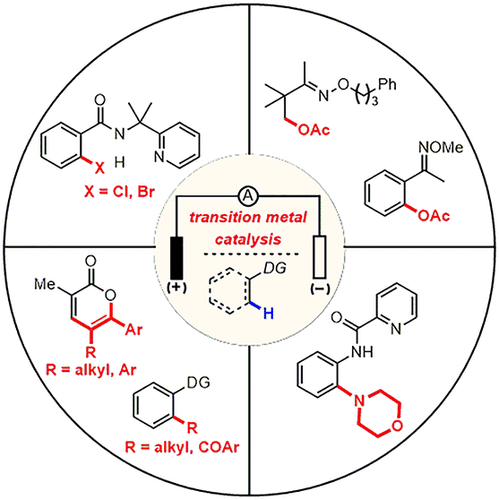
Electrochemical synthesis of organic compounds has emerged as an attractive and environmentally benign alternative to conventional approaches for oxidation and reduction of organic compounds that utilizes electric current instead of chemical oxidants and reductants. As such, many useful transformations have been developed, including the Kolbe reaction, the Simons fluorination process, the Monsanto adiponitrile process, and the Shono oxidation, to name a few. Electrochemical C-H functionalization represents one of the most promising reaction types among many electrochemical transformations, since this process avoids prefunctionalization of substrates and provides novel retrosynthetic disconnections. However, site-selective anodic oxidation of C-H bonds is still a fundamental challenge due to the high oxidation potentials of C-H bonds compared to organic solvents and common functional groups. To overcome this issue, indirect electrolysis via the action of a mediator (a redox catalyst) is regularly employed, by which the selectivity can be controlled following reaction of said mediator with the substrate. Since the redox potentials of transition metal complexes can be easily tuned by modification of the ligand, the synergistic use of electrochemistry and transition metal catalysis to achieve site-selective C-H functionalization is an attractive strategy. In this Account, we summarize and contextualize our recent efforts toward transition metal-catalyzed electrochemical C-H functionalization proximal to a suitable directing group. We have developed C-H oxygenation, acylation, alkylation, and halogenation reactions in which a Pd(II) species is oxidized to a Pd(III) or Pd(IV) intermediate by anodic oxidation, followed by reductive elimination to form the corresponding C-O, C-C, and C-X bonds. Importantly, improved monofunctionalization selectivity is achieved in the Pd-catalyzed C(sp3)-H oxygenation compared to conventional approaches using PhI(OAc)2 as the chemical oxidant. Physical separators are sometimes used to prevent the electrochemical deposition of Pd black on the cathode resulting from reduction of high valent Pd species. We skirted this issue through the development a Cu-catalyzed electrochemical C(sp2)-H amination using n-Bu4NI as a redox cocatalyst in an undivided cell. In addition, we developed Ir-catalyzed electrochemical vinylic C-H functionalization of acrylic acids with alkynes in an undivided cell, affording various substituted α-pyrones in good to excellent yield. More importantly, chemical oxidants, including Ag2CO3, Cu(OAc)2, and PhI(OAc)2, resulted in much lower yields in the absence of electrical current under otherwise identical conditions. As elaborated below, progress in the area of electrochemical transition metal-catalyzed synthesis provides an effective platform for environmentally friendly and sustainable selective chemical transformations.
中文翻译:

通过协同使用电化学和过渡金属催化进行的位点选择性CH功能化。
有机化合物的电化学合成已经成为对使用电流代替化学氧化剂和还原剂的有机化合物进行氧化和还原的常规方法的一种有吸引力且对环境无害的替代方法。因此,已经开发出许多有用的转化,包括Kolbe反应,Simons氟化过程,孟山都己二腈过程和Shono氧化等。电化学CH官能化代表了许多电化学转化中最有希望的反应类型之一,因为该过程避免了底物的预官能化并提供了新颖的逆合成断开作用。然而,由于CH键与有机溶剂和常见官能团相比具有较高的氧化电位,因此CH键的位点选择性阳极氧化仍然是一项基本挑战。为了克服该问题,通常采用通过介体(氧化还原催化剂)的作用进行间接电解,通过该间接电解可以在所述介体与底物反应之后控制选择性。由于过渡金属配合物的氧化还原电势可以通过配体的修饰而容易地调节,因此电化学和过渡金属催化的协同使用以实现位点选择性CH官能化是一种有吸引力的策略。在此报告中,我们总结并总结了我们最近在过渡金属催化的电化学CH官能化附近合适的导向基团方面所做的努力。我们已经开发了CH氧化,酰化,烷基化和卤化反应,其中Pd(II)物种通过阳极氧化被氧化为Pd(III)或Pd(IV)中间体,然后还原消除形成相应的CO,CC和CX键。重要的是,与使用PhI(OAc)2作为化学氧化剂的常规方法相比,在Pd催化的C(sp3)-H氧合中实现了改进的单官能化选择性。有时会使用物理隔板来防止由于高价Pd物种的还原而导致Pd黑在阴极上的电化学沉积。我们通过开发使用n-Bu4NI作为未分裂电池中的氧化还原助催化剂的Cu催化电化学C(sp2)-H胺化来解决这个问题。此外,我们在一个不分开的电池中开发了用炔烃对丙烯酸进行Ir催化的电化学乙烯基CH官能化,从而以良好或优异的收率提供了各种取代的α-吡喃酮。更重要的是,在其他条件相同的情况下,在没有电流的情况下,包括Ag2CO3,Cu(OAc)2和PhI(OAc)2在内的化学氧化剂导致产量大大降低。如下所述,电化学过渡金属催化合成领域的进展为环境友好和可持续的选择性化学转化提供了有效的平台。在其他条件相同的情况下,在没有电流的情况下,导致产量大大降低。如下所述,电化学过渡金属催化合成领域的进展为环境友好和可持续的选择性化学转化提供了有效的平台。在其他条件相同的情况下,在没有电流的情况下,导致产量大大降低。如下所述,电化学过渡金属催化合成领域的进展为环境友好和可持续的选择性化学转化提供了有效的平台。
更新日期:2020-01-15
中文翻译:

通过协同使用电化学和过渡金属催化进行的位点选择性CH功能化。
有机化合物的电化学合成已经成为对使用电流代替化学氧化剂和还原剂的有机化合物进行氧化和还原的常规方法的一种有吸引力且对环境无害的替代方法。因此,已经开发出许多有用的转化,包括Kolbe反应,Simons氟化过程,孟山都己二腈过程和Shono氧化等。电化学CH官能化代表了许多电化学转化中最有希望的反应类型之一,因为该过程避免了底物的预官能化并提供了新颖的逆合成断开作用。然而,由于CH键与有机溶剂和常见官能团相比具有较高的氧化电位,因此CH键的位点选择性阳极氧化仍然是一项基本挑战。为了克服该问题,通常采用通过介体(氧化还原催化剂)的作用进行间接电解,通过该间接电解可以在所述介体与底物反应之后控制选择性。由于过渡金属配合物的氧化还原电势可以通过配体的修饰而容易地调节,因此电化学和过渡金属催化的协同使用以实现位点选择性CH官能化是一种有吸引力的策略。在此报告中,我们总结并总结了我们最近在过渡金属催化的电化学CH官能化附近合适的导向基团方面所做的努力。我们已经开发了CH氧化,酰化,烷基化和卤化反应,其中Pd(II)物种通过阳极氧化被氧化为Pd(III)或Pd(IV)中间体,然后还原消除形成相应的CO,CC和CX键。重要的是,与使用PhI(OAc)2作为化学氧化剂的常规方法相比,在Pd催化的C(sp3)-H氧合中实现了改进的单官能化选择性。有时会使用物理隔板来防止由于高价Pd物种的还原而导致Pd黑在阴极上的电化学沉积。我们通过开发使用n-Bu4NI作为未分裂电池中的氧化还原助催化剂的Cu催化电化学C(sp2)-H胺化来解决这个问题。此外,我们在一个不分开的电池中开发了用炔烃对丙烯酸进行Ir催化的电化学乙烯基CH官能化,从而以良好或优异的收率提供了各种取代的α-吡喃酮。更重要的是,在其他条件相同的情况下,在没有电流的情况下,包括Ag2CO3,Cu(OAc)2和PhI(OAc)2在内的化学氧化剂导致产量大大降低。如下所述,电化学过渡金属催化合成领域的进展为环境友好和可持续的选择性化学转化提供了有效的平台。在其他条件相同的情况下,在没有电流的情况下,导致产量大大降低。如下所述,电化学过渡金属催化合成领域的进展为环境友好和可持续的选择性化学转化提供了有效的平台。在其他条件相同的情况下,在没有电流的情况下,导致产量大大降低。如下所述,电化学过渡金属催化合成领域的进展为环境友好和可持续的选择性化学转化提供了有效的平台。


























 京公网安备 11010802027423号
京公网安备 11010802027423号