当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
ZnCl 2 -Catalyzed [4+1] Annulation of Alkylthio-Substituted Enaminones and Enaminothiones with Sulfur Ylides.
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2020-01-15 , DOI: 10.1002/chem.201905483 Yuan He 1, 2 , Jiang Lou 1, 2 , Yong-Gui Zhou 1 , Zhengkun Yu 1, 3
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2020-01-15 , DOI: 10.1002/chem.201905483 Yuan He 1, 2 , Jiang Lou 1, 2 , Yong-Gui Zhou 1 , Zhengkun Yu 1, 3
Affiliation
|
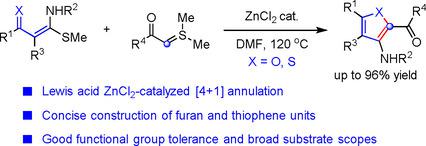
Concise construction of furan and thiophene units has played an important role in the synthesis of potentially bioactive compounds and functional materials. Herein, we report an efficient Lewis acid ZnCl 2 -catalyzed [4+1] annulation of alkylthio-substituted enami-nones, that is, α -oxo ketene N,S -acetals, with sulfur ylides, affording 2-acyl-3-aminofuran derivatives. In a similar fashion, [4+1] annulation of the corresponding enaminothiones, that is, α -thioxo ketene N,S -acetals, with sulfur ylides efficiently proceeded to give multisubstituted 3-aminothiophenes. This method features wide substrate scopes as well as broad functional group tolerance, offering a concise route to highly functionalized furans and thiophenes.
中文翻译:

ZnCl 2催化[4 + 1]烷硫基取代的烯胺酮和烯胺硫酮与硫叶立德的环化。
呋喃和噻吩单元的简洁结构在潜在生物活性化合物和功能材料的合成中发挥了重要作用。在本文中,我们报告了一种有效的路易斯酸ZnCl 2催化的烷硫基取代的烯胺酮(即α-氧代烯酮N,S-乙缩醛)与硫的酰基化物的[4 + 1]环化反应,得到2-酰基-3-氨基呋喃衍生物。以类似的方式,相应的烯氨基噻酮,即α-硫代乙烯酮N,S-乙缩醛与硫酰化物的[4 + 1]环化有效地产生了多取代的3-氨基噻吩。该方法具有广泛的底物范围和宽泛的官能团耐受性,为获得高度官能化的呋喃和噻吩提供了一条简洁的途径。
更新日期:2020-03-24
中文翻译:

ZnCl 2催化[4 + 1]烷硫基取代的烯胺酮和烯胺硫酮与硫叶立德的环化。
呋喃和噻吩单元的简洁结构在潜在生物活性化合物和功能材料的合成中发挥了重要作用。在本文中,我们报告了一种有效的路易斯酸ZnCl 2催化的烷硫基取代的烯胺酮(即α-氧代烯酮N,S-乙缩醛)与硫的酰基化物的[4 + 1]环化反应,得到2-酰基-3-氨基呋喃衍生物。以类似的方式,相应的烯氨基噻酮,即α-硫代乙烯酮N,S-乙缩醛与硫酰化物的[4 + 1]环化有效地产生了多取代的3-氨基噻吩。该方法具有广泛的底物范围和宽泛的官能团耐受性,为获得高度官能化的呋喃和噻吩提供了一条简洁的途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号