当前位置:
X-MOL 学术
›
Commun. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Combinatorial allosteric modulation of agonist response in a self-interacting G-protein coupled receptor.
Communications Biology ( IF 5.9 ) Pub Date : 2020-01-15 , DOI: 10.1038/s42003-020-0752-4 Marco Patrone 1 , Eugenia Cammarota 2 , Valeria Berno 3 , Paola Tornaghi 1 , Davide Mazza 2 , Massimo Degano 1
Communications Biology ( IF 5.9 ) Pub Date : 2020-01-15 , DOI: 10.1038/s42003-020-0752-4 Marco Patrone 1 , Eugenia Cammarota 2 , Valeria Berno 3 , Paola Tornaghi 1 , Davide Mazza 2 , Massimo Degano 1
Affiliation
|
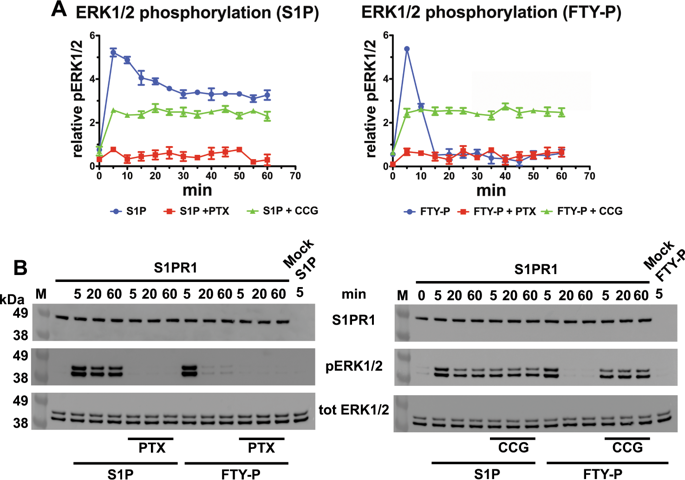
The structural plasticity of G-protein coupled receptors (GPCRs) enables the long-range transmission of conformational changes induced by specific orthosteric site ligands and other pleiotropic factors. Here, we demonstrate that the ligand binding cavity in the sphingosine 1-phosphate receptor S1PR1, a class A GPCR, is in allosteric communication with both the β-arrestin-binding C-terminal tail, and a receptor surface involved in oligomerization. We show that S1PR1 oligomers are required for full response to different agonists and ligand-specific association with arrestins, dictating the downstream signalling kinetics. We reveal that the active form of the immunomodulatory drug fingolimod, FTY720-P, selectively harnesses both these intramolecular networks to efficiently recruit β-arrestins in a stable interaction with the receptor, promoting deep S1PR1 internalization and simultaneously abrogating ERK1/2 phosphorylation. Our results define a molecular basis for the efficacy of fingolimod for people with multiple sclerosis, and attest that GPCR signalling can be further fine-tuned by the oligomeric state.
中文翻译:

在自我相互作用的G蛋白偶联受体中激动剂反应的组合变构调节。
G蛋白偶联受体(GPCR)的结构可塑性使特定的正构位点配体和其他多效性因子引起的构象变化的远距离传递成为可能。在这里,我们证明了鞘氨醇1-磷酸受体S1PR1(A类GPCR)中的配体结合腔与β-arrestin结合的C末端尾部和参与寡聚的受体表面都处于变构联系。我们表明,S1PR1寡聚体对于完全不同的激动剂和与抑制蛋白的配体特异性缔合具有完全反应所必需,从而决定了下游信号传导动力学。我们发现,免疫调节药物芬戈莫德FTY720-P的活性形式选择性地利用了这些分子内网络,以有效地募集与受体稳定相互作用的β-arrestin,促进深S1PR1内在化并同时废除ERK1 / 2磷酸化。我们的结果为芬戈莫德对多发性硬化症患者的疗效定义了分子基础,并证明GPCR信号传导可通过寡聚状态进一步微调。
更新日期:2020-01-15
中文翻译:

在自我相互作用的G蛋白偶联受体中激动剂反应的组合变构调节。
G蛋白偶联受体(GPCR)的结构可塑性使特定的正构位点配体和其他多效性因子引起的构象变化的远距离传递成为可能。在这里,我们证明了鞘氨醇1-磷酸受体S1PR1(A类GPCR)中的配体结合腔与β-arrestin结合的C末端尾部和参与寡聚的受体表面都处于变构联系。我们表明,S1PR1寡聚体对于完全不同的激动剂和与抑制蛋白的配体特异性缔合具有完全反应所必需,从而决定了下游信号传导动力学。我们发现,免疫调节药物芬戈莫德FTY720-P的活性形式选择性地利用了这些分子内网络,以有效地募集与受体稳定相互作用的β-arrestin,促进深S1PR1内在化并同时废除ERK1 / 2磷酸化。我们的结果为芬戈莫德对多发性硬化症患者的疗效定义了分子基础,并证明GPCR信号传导可通过寡聚状态进一步微调。



























 京公网安备 11010802027423号
京公网安备 11010802027423号