当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The First Structure of an Active Mammalian dCTPase and its Complexes With Substrate Analogs and Products.
Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-01-15 , DOI: 10.1016/j.jmb.2020.01.005 Emma Scaletti 1 , Magnus Claesson 2 , Thomas Helleday 3 , Ann-Sofie Jemth 4 , Pål Stenmark 1
Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-01-15 , DOI: 10.1016/j.jmb.2020.01.005 Emma Scaletti 1 , Magnus Claesson 2 , Thomas Helleday 3 , Ann-Sofie Jemth 4 , Pål Stenmark 1
Affiliation
|
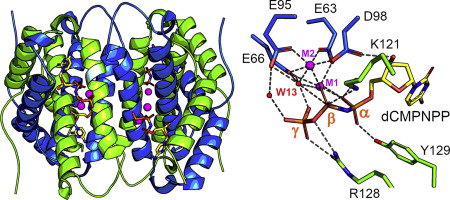
Precise regulation of dNTPs within the cellular nucleotide pool is essential for high accuracy of DNA replication and is critical for retaining the genomic integrity. Recently, human dCTPase (deoxycytidine triphosphatase), also known as DCTPP1 (human all-alpha dCTP pyrophosphatase 1), has been revealed to be a key player in the balance of pyrimidine nucleotide concentrations within cells, with DCTPP1 deficiency causing DNA damage and genetic instability in both chromosomal and mitochondrial DNA. DCTPP1 also exhibits an additional "house cleaning" function as it has been shown to be highly active against modified cytidine triphosphates, such as 5-methyl-dCTP, which, if incorrectly incorporated into DNA can introduce undesirable epigenetic marking. To date, structural studies of mammalian dCTPase have been limited to inactive constructs, which do not provide information regarding the catalytic mechanism of this important enzyme. We present here the first structures of an active mammalian dCTPase from M. musculus in complex with the nonhydrolyzable substrate analog dCMPNPP and the products 5-Me-dCMP and dCMP. These structures provide clear insights into substrate binding and catalysis and clearly elucidate why previous structures of mammalian dCTPase were catalytically inactive. The overall structure of M. musculus dCTPase is highly similar to enzymes from the all-alpha NTP phosphohydrolase superfamily. Comparison of M. musculus dCTPase with homologs from a diverse range of mammals, including humans, shows that the residues, which contribute to substrate recognition, are entirely conserved, further supporting the importance of this enzyme in the protection of genomic integrity in mammalian cells.
中文翻译:

活性哺乳动物dCTPase的第一个结构及其与底物类似物和产物的复合物。
精确调节细胞核苷酸池中的dNTP对DNA复制的高精度至关重要,对于保持基因组完整性至关重要。最近,人dCTPase(脱氧胞苷三磷酸酶),也称为DCTPP1(人全αdCTP焦磷酸酶1),已被证明是细胞内嘧啶核苷酸浓度平衡的关键因素,DCTPP1缺乏会引起DNA损伤和遗传不稳定。在染色体和线粒体DNA中都存在 DCTPP1还具有额外的“室内清洁”功能,因为它已显示出对修饰的胞苷三磷酸(如5-甲基-dCTP)具有高活性,如果将其错误地掺入DNA中,则会引入不良的表观遗传标记。迄今为止,哺乳动物dCTPase的结构研究仅限于非活性构建体,没有提供有关此重要酶催化机理的信息。我们在此介绍了来自小家鼠的活性哺乳动物dCTPase的第一个结构,该结构与不可水解的底物类似物dCMPNPP和产物5-Me-dCMP和dCMP复杂。这些结构提供了对底物结合和催化作用的清晰见解,并清楚阐明了为什么哺乳动物dCTPase的先前结构没有催化活性。小家鼠dCTPase的总体结构与全αNTP磷酸水解酶超家族的酶高度相似。将小家鼠dCTPase与包括人类在内的多种哺乳动物的同源物进行比较,结果表明,有助于底物识别的残基是完全保守的,
更新日期:2020-01-15
中文翻译:

活性哺乳动物dCTPase的第一个结构及其与底物类似物和产物的复合物。
精确调节细胞核苷酸池中的dNTP对DNA复制的高精度至关重要,对于保持基因组完整性至关重要。最近,人dCTPase(脱氧胞苷三磷酸酶),也称为DCTPP1(人全αdCTP焦磷酸酶1),已被证明是细胞内嘧啶核苷酸浓度平衡的关键因素,DCTPP1缺乏会引起DNA损伤和遗传不稳定。在染色体和线粒体DNA中都存在 DCTPP1还具有额外的“室内清洁”功能,因为它已显示出对修饰的胞苷三磷酸(如5-甲基-dCTP)具有高活性,如果将其错误地掺入DNA中,则会引入不良的表观遗传标记。迄今为止,哺乳动物dCTPase的结构研究仅限于非活性构建体,没有提供有关此重要酶催化机理的信息。我们在此介绍了来自小家鼠的活性哺乳动物dCTPase的第一个结构,该结构与不可水解的底物类似物dCMPNPP和产物5-Me-dCMP和dCMP复杂。这些结构提供了对底物结合和催化作用的清晰见解,并清楚阐明了为什么哺乳动物dCTPase的先前结构没有催化活性。小家鼠dCTPase的总体结构与全αNTP磷酸水解酶超家族的酶高度相似。将小家鼠dCTPase与包括人类在内的多种哺乳动物的同源物进行比较,结果表明,有助于底物识别的残基是完全保守的,


























 京公网安备 11010802027423号
京公网安备 11010802027423号