Nature Reviews Chemistry ( IF 36.3 ) Pub Date : 2020-01-14 , DOI: 10.1038/s41570-019-0158-3 Greeshma Gadikota 1
|
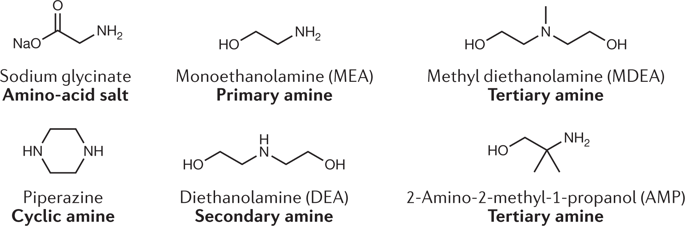
There is a need to capture, convert and store CO2 by atom-efficient and energy-efficient pathways that use as few process configurations as possible. This need has motivated studies into multiphase reaction chemistries and this Review describes two such approaches in the context of carbon mineralization. The first approach uses aqueous alkaline solutions containing amine nucleophiles that capture CO2 and eventually convert it into calcium and magnesium carbonates, thereby regenerating the nucleophiles. Gas–liquid–solid and liquid–solid configurations of these reactions are explored. The second approach combines silicates such as CaSiO3 or Mg2SiO4 with CO and H2O from the water-gas shift reaction to give H2 and calcium or magnesium carbonates. Coupling carbonate formation to the water-gas shift reaction shifts the latter equilibrium to afford more H2 as part of a single-step catalytic approach to carbon mineralization. These pathways exploit the vast abundance of alkaline resources, including naturally occurring silicates and alkaline industrial residues. However, simple stoichiometries belie the complex, multiphase nature of the reactions, predictive control of which presents a scientific opportunity and challenge. This Review describes this multiphase chemistry and the knowledge gaps that need to be addressed to achieve ‘step-change’ advancements in the reactive separation of CO2 by carbon mineralization.
中文翻译:

用于CO 2 反应分离和H 2 定向合成的多相碳矿化
需要通过使用尽可能少的工艺配置的原子高效和节能途径来捕获、转化和储存 CO 2 。这种需求推动了对多相反应化学的研究,本综述描述了碳矿化背景下的两种此类方法。第一种方法使用含有胺亲核试剂的碱性水溶液捕获 CO 2并最终将其转化为碳酸钙和碳酸镁,从而使亲核试剂再生。探索了这些反应的气-液-固和液-固构型。第二种方法将硅酸盐(例如 CaSiO 3或 Mg 2 SiO 4 )与 CO 和 H 2结合起来来自水煤气变换反应的 O 生成 H 2和碳酸钙或碳酸镁。将碳酸盐的形成与水煤气变换反应相结合,改变了后者的平衡以提供更多的 H 2作为碳矿化单步催化方法的一部分。这些途径利用了大量的碱性资源,包括天然存在的硅酸盐和碱性工业残留物。然而,简单的化学计量掩盖了反应的复杂、多相性质,其预测控制提出了科学机遇和挑战。这篇综述描述了这种多相化学以及需要解决的知识差距,以实现通过碳矿化反应分离 CO 2的“阶跃式”进步。



























 京公网安备 11010802027423号
京公网安备 11010802027423号