当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Probing the Nongeneralized Amyloid Inhibitory Mechanism of Hydrophobic Chaperone.
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-01-22 , DOI: 10.1021/acschemneuro.9b00593 Aiman Masroor 1 , Nida Zaidi 1 , Tajalli Ilm Chandel 1 , Zoha Aqueel 1 , Sadia Malik 1 , Rizwan Hasan Khan 1
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-01-22 , DOI: 10.1021/acschemneuro.9b00593 Aiman Masroor 1 , Nida Zaidi 1 , Tajalli Ilm Chandel 1 , Zoha Aqueel 1 , Sadia Malik 1 , Rizwan Hasan Khan 1
Affiliation
|
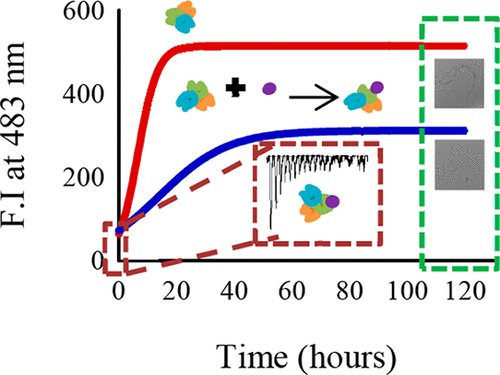
Increasing prevalence of protein misfolding disorders urges the search for effective therapies. Although several antiaggregation molecules have been identified, their molecular process of aggregation and clinical trials are underway. The present study is focused on the mechanism through which phenyl butyrate (PB), a chemical chaperone, triggers inhibition of human serum albumin (HSA) fibrillation. Turbidity and Rayleigh light scattering (RLS) measurements reveal the marked presence of aggregates in HSA that were confirmed as amyloid fibrils by thioflavin T (ThT) and Congo red (CR) and were subsequently inhibited by PB in a dose dependent manner. ThT fluorescence kinetics reveals a decrease in the apparent rate constant, Kapp, in the presence of PB without triggering a lag phase in HSA suggesting PB's interference with the elongation phase. Dynamic light scattering (DLS) results display a reduction in the aggregate size in the presence of PB. Isothermal titration calorimetry (ITC) data reveals strong binding of PB at site II both at 25 °C (Kb ≈ 1.94 × 105 M-1) and 65 °C (Kb ≈ 2.90 × 104 M-1), mediated by hydrogen bonding. Overall, our finding establishes that PB stabilizes partially unfolded HSA molecules through hydrogen bonding, thereby preventing establishment of hydrogen bonds between them and hindering their progression into amyloid fibrils. This is in contrast to its chaperone effect manifested with other proteins.
中文翻译:

探索疏水伴侣蛋白的非一般淀粉样蛋白抑制机制。
蛋白质错误折叠障碍的患病率上升,促使人们寻求有效的疗法。尽管已经确定了几种抗聚集分子,但它们的聚集过程和临床试验仍在进行中。本研究的重点是机制,化学伴侣伴侣丁酸苯酯(PB)触发抑制人血清白蛋白(HSA)颤动。浊度和瑞利光散射(RLS)测量揭示了HSA中聚集体的明显存在,这些聚集体被硫黄素T(ThT)和刚果红(CR)确认为淀粉样原纤维,随后被PB以剂量依赖性方式抑制。ThT荧光动力学揭示了在存在PB的情况下表观速率常数Kapp的降低,而没有触发HSA的滞后阶段,表明PB干扰了延伸相。动态光散射(DLS)结果显示,在PB存在下,聚集体尺寸减小。等温滴定量热法(ITC)数据显示,在25°C(Kb≈1.94×105 M-1)和65°C(Kb≈2.90×104 M-1)处,II位的PB都通过氢键牢固结合。总体而言,我们的发现建立了PB通过氢键稳定部分展开的HSA分子的作用,从而防止了它们之间氢键的建立并阻碍了它们发展为淀粉样蛋白原纤维。这与其他蛋白表现出的伴侣作用相反。氢键介导 总体而言,我们的发现建立了PB通过氢键稳定部分展开的HSA分子的作用,从而防止了它们之间氢键的建立并阻碍了它们发展为淀粉样蛋白原纤维。这与其他蛋白表现出的伴侣作用相反。氢键介导 总体而言,我们的发现建立了PB通过氢键稳定部分展开的HSA分子的作用,从而防止了它们之间氢键的建立并阻碍了它们发展为淀粉样蛋白原纤维。这与其他蛋白表现出的伴侣作用相反。
更新日期:2020-01-23
中文翻译:

探索疏水伴侣蛋白的非一般淀粉样蛋白抑制机制。
蛋白质错误折叠障碍的患病率上升,促使人们寻求有效的疗法。尽管已经确定了几种抗聚集分子,但它们的聚集过程和临床试验仍在进行中。本研究的重点是机制,化学伴侣伴侣丁酸苯酯(PB)触发抑制人血清白蛋白(HSA)颤动。浊度和瑞利光散射(RLS)测量揭示了HSA中聚集体的明显存在,这些聚集体被硫黄素T(ThT)和刚果红(CR)确认为淀粉样原纤维,随后被PB以剂量依赖性方式抑制。ThT荧光动力学揭示了在存在PB的情况下表观速率常数Kapp的降低,而没有触发HSA的滞后阶段,表明PB干扰了延伸相。动态光散射(DLS)结果显示,在PB存在下,聚集体尺寸减小。等温滴定量热法(ITC)数据显示,在25°C(Kb≈1.94×105 M-1)和65°C(Kb≈2.90×104 M-1)处,II位的PB都通过氢键牢固结合。总体而言,我们的发现建立了PB通过氢键稳定部分展开的HSA分子的作用,从而防止了它们之间氢键的建立并阻碍了它们发展为淀粉样蛋白原纤维。这与其他蛋白表现出的伴侣作用相反。氢键介导 总体而言,我们的发现建立了PB通过氢键稳定部分展开的HSA分子的作用,从而防止了它们之间氢键的建立并阻碍了它们发展为淀粉样蛋白原纤维。这与其他蛋白表现出的伴侣作用相反。氢键介导 总体而言,我们的发现建立了PB通过氢键稳定部分展开的HSA分子的作用,从而防止了它们之间氢键的建立并阻碍了它们发展为淀粉样蛋白原纤维。这与其他蛋白表现出的伴侣作用相反。


























 京公网安备 11010802027423号
京公网安备 11010802027423号