Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystal Chemistry, Band-Gap Red Shift, and Electrocatalytic Activity of Iron-Doped Gallium Oxide Ceramics.
ACS Omega ( IF 4.1 ) Pub Date : 2019-12-27 , DOI: 10.1021/acsomega.9b01604 Bandi Mallesham 1 , Swadipta Roy 1, 1, 2 , Saptasree Bose 1 , Aruna N Nair 1 , Sreeprasad Sreenivasan 1 , Vaithiyalingam Shutthanandan 2 , Chintalapalle V Ramana 1
ACS Omega ( IF 4.1 ) Pub Date : 2019-12-27 , DOI: 10.1021/acsomega.9b01604 Bandi Mallesham 1 , Swadipta Roy 1, 1, 2 , Saptasree Bose 1 , Aruna N Nair 1 , Sreeprasad Sreenivasan 1 , Vaithiyalingam Shutthanandan 2 , Chintalapalle V Ramana 1
Affiliation
|
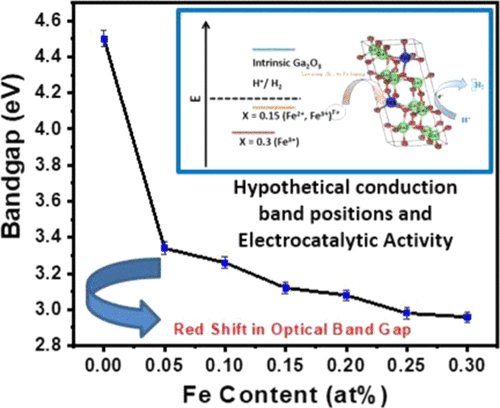
This work for the first time unfurls the fundamental mechanisms and sets the stage for an approach to derive electrocatalytic activity, which is otherwise not possible, in a traditionally known wide band-gap oxide material. Specifically, we report on the tunable optical properties, in terms of wide spectral selectivity and red-shifted band gap, and electrocatalytic behavior of iron (Fe)-doped gallium oxide (β-Ga2O3) model system. X-ray diffraction (XRD) studies of sintered Ga2-x Fe x O3 (GFO) (0.0 ≤ x ≤ 0.3) compounds provide evidence for the Fe3+ substitution at Ga3+ site without any secondary phase formation. Rietveld refinement of XRD patterns reveals that the GFO compounds crystallize in monoclinic crystal symmetry with a C2/m space group. The electronic structure of the GFO compounds probed using X-ray photoelectron spectroscopy data reveals that at lower concentrations, Fe exhibits mixed chemical valence states (Fe3+, Fe2+), whereas single chemical valence state (Fe3+) is evident for higher Fe content (x = 0.20-0.30). The optical absorption spectra reveal a significant red shift in the optical band gap with Fe doping. The origin of the significant red shift even at low concentrations of Fe (x = 0.05) is attributed to the strong sp-d exchange interaction originated from the 3d5 electrons of Fe3+. The optical absorption edge observed at ≈450 nm with lower intensity is the characteristic of Fe-doped compounds associated with Fe3+-Fe3+ double-excitation process. Coupled with an optical band-gap red shift, electrocatalytic studies of GFO compounds reveal that, interestingly, Fe-doped Ga2O3 compound exhibits electrocatalytic activity in contrast to intrinsic Ga2O3. Fe-doped samples (GFO) demonstrated appreciable electrocatalytic activity toward the generation of H2 through electrocatalytic water splitting. An onset potential and Tafel slope of GFO compounds include ∼900 mV, ∼210 mV dec-1 (x = 0.15) and ∼1036 mV, ∼290 mV dec-1 (x = 0.30), respectively. The electrocatalytic activity of Fe-doped Ga-oxide compounds is attributed to the cumulative effect of different mechanisms such as doping resulting in new catalytic centers, enhanced conductivity, and electron mobility. Hence, in this report, for the first time, we explored a new pathway; the electrocatalytic behavior of Fe-doped Ga2O3 resulted due to Fe chemical states and red shift in the optical band gap. The implications derived from this work may be applicable to a large class of compounds, and further options may be available to design functional materials for electrocatalytic energy production.
中文翻译:

铁掺杂的镓氧化物陶瓷的晶体化学,带隙红移和电催化活性。
这项工作首次揭示了基本机理,并为获得电催化活性的方法奠定了基础,而这在传统上已知的宽带隙氧化物材料中是不可能的。具体来说,我们就宽光谱选择性和红移带隙以及可掺杂铁(Fe)的氧化镓(β-Ga2O3)模型系统的电催化行为等方面报道了可调光学性质。烧结的Ga2-x Fe x O3(GFO)(0.0≤x≤0.3)化合物的X射线衍射(XRD)研究提供了在Ga3 +位上没有任何次级相形成的Fe3 +取代的证据。XRD图案的Rietveld精炼显示,GFO化合物以C2 / m空间群的单斜晶体对称结晶。使用X射线光电子能谱数据探测的GFO化合物的电子结构表明,在较低浓度下,Fe表现出混合的化学价态(Fe3 +,Fe2 +),而对于较高的Fe含量则明显表现出单一的化学价态(Fe3 +)(x = 0.20-0.30)。光学吸收光谱显示出,随着Fe的掺杂,光学带隙出现明显的红移。即使在低浓度的Fe(x = 0.05)下,明显的红移的起源也归因于源自Fe3 +的3d5电子的强sp-d交换相互作用。在≈450nm处以较低的强度观察到的光吸收边缘是与Fe3 + -Fe3 +双重激发过程相关的Fe掺杂化合物的特征。结合光学带隙红移,GFO化合物的电催化研究表明,有趣的是,与固有的Ga2O3相比,掺Fe的Ga2O3化合物具有电催化活性。掺杂铁的样品(GFO)对通过电催化水分解产生的H2具有明显的电催化活性。GFO化合物的起始电位和Tafel斜率分别为〜900 mV,〜210 mV dec-1(x = 0.15)和〜1036 mV,〜290 mV dec-1(x = 0.30)。Fe掺杂的Ga氧化物的电催化活性归因于不同机理的累积效应,例如掺杂导致新的催化中心,增强的电导率和电子迁移率。因此,在本报告中,我们首次探索了一条新途径。Fe掺杂的Ga2O3的电催化行为归因于Fe的化学态和光学带隙中的红移。
更新日期:2020-01-14
中文翻译:

铁掺杂的镓氧化物陶瓷的晶体化学,带隙红移和电催化活性。
这项工作首次揭示了基本机理,并为获得电催化活性的方法奠定了基础,而这在传统上已知的宽带隙氧化物材料中是不可能的。具体来说,我们就宽光谱选择性和红移带隙以及可掺杂铁(Fe)的氧化镓(β-Ga2O3)模型系统的电催化行为等方面报道了可调光学性质。烧结的Ga2-x Fe x O3(GFO)(0.0≤x≤0.3)化合物的X射线衍射(XRD)研究提供了在Ga3 +位上没有任何次级相形成的Fe3 +取代的证据。XRD图案的Rietveld精炼显示,GFO化合物以C2 / m空间群的单斜晶体对称结晶。使用X射线光电子能谱数据探测的GFO化合物的电子结构表明,在较低浓度下,Fe表现出混合的化学价态(Fe3 +,Fe2 +),而对于较高的Fe含量则明显表现出单一的化学价态(Fe3 +)(x = 0.20-0.30)。光学吸收光谱显示出,随着Fe的掺杂,光学带隙出现明显的红移。即使在低浓度的Fe(x = 0.05)下,明显的红移的起源也归因于源自Fe3 +的3d5电子的强sp-d交换相互作用。在≈450nm处以较低的强度观察到的光吸收边缘是与Fe3 + -Fe3 +双重激发过程相关的Fe掺杂化合物的特征。结合光学带隙红移,GFO化合物的电催化研究表明,有趣的是,与固有的Ga2O3相比,掺Fe的Ga2O3化合物具有电催化活性。掺杂铁的样品(GFO)对通过电催化水分解产生的H2具有明显的电催化活性。GFO化合物的起始电位和Tafel斜率分别为〜900 mV,〜210 mV dec-1(x = 0.15)和〜1036 mV,〜290 mV dec-1(x = 0.30)。Fe掺杂的Ga氧化物的电催化活性归因于不同机理的累积效应,例如掺杂导致新的催化中心,增强的电导率和电子迁移率。因此,在本报告中,我们首次探索了一条新途径。Fe掺杂的Ga2O3的电催化行为归因于Fe的化学态和光学带隙中的红移。



























 京公网安备 11010802027423号
京公网安备 11010802027423号