当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
dGMP Binding to Thymidylate Kinase from Plasmodium falciparum Shows Half-Site Binding and Induces Protein Dynamics at the Dimer Interface.
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-14 , DOI: 10.1021/acs.biochem.9b00898 Mengshen David Chen 1 , Ian J Fucci 1 , Kaustubh Sinha 1 , Gordon S Rule 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-14 , DOI: 10.1021/acs.biochem.9b00898 Mengshen David Chen 1 , Ian J Fucci 1 , Kaustubh Sinha 1 , Gordon S Rule 1
Affiliation
|
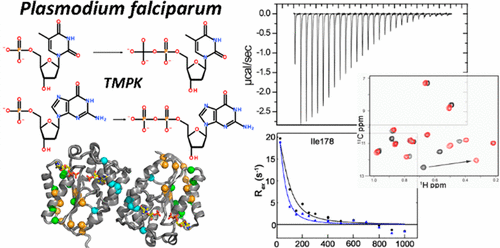
Plasmodium falciparum thymidylate kinase (PfTMK) is an essential enzyme for the growth of the organism because of its critical role in the de novo synthesis of deoxythymidine 5'-diphosphate (TDP), a precursor for TTP that is required for DNA replication and repair. The kinetics, thermodynamic parameters, and substrate binding properties of PfTMK for TMP, dGMP, ADP, and ATP were measured and characterized by steady-state kinetics and a combination of isothermal titration calorimetry, tryptophan fluorescence titration, and NMR. Mutational studies were performed to investigate residues that contribute to the unique ability of PfTMK to also utilize dGMP as a substrate. Isothermal titration calorimetry experiments revealed that dGMP binding exhibits a unique half-site binding mechanism. The occlusion of the empty site in the dGMP complex is supported by molecular mechanics calculations. Relaxation dispersion experiments show that the dGMP and enzyme complex is more dynamic at the dimer interface than the TMP complex on the μs-ms time scale. The unique properties of dGMP binding need to be considered in the design of guanosine-based PfTMK-specific inhibitors.
中文翻译:

dGMP与恶性疟原虫的胸苷酸激酶的结合显示半位点结合并在二聚体界面诱导蛋白质动力学。
恶性疟原虫胸苷酸激酶(PfTMK)是生物生长的必需酶,因为它在脱氧胸苷5'-二磷酸(TDP)的从头合成中起着关键作用,脱氧胸苷是5'-二磷酸(TDP)的前体,是DNA复制和修复所需的TTP前体。测量并分析了PfTMK对TMP,dGMP,ADP和ATP的动力学,热力学参数和底物结合特性,并通过稳态动力学以及等温滴定量热法,色氨酸荧光滴定和NMR的组合进行了表征。进行了突变研究,以研究有助于PfTMK也利用dGMP作为底物的独特能力的残基。等温滴定量热法实验表明,dGMP结合表现出独特的半位点结合机制。分子力学计算支持dGMP复合物中空位的封闭。弛豫分散实验表明,在μs-ms时间尺度上,dGMP和酶复合物在二聚体界面上比TMP复合物更动态。在设计基于鸟苷的PfTMK特异性抑制剂时,必须考虑dGMP结合的独特特性。
更新日期:2020-01-14
中文翻译:

dGMP与恶性疟原虫的胸苷酸激酶的结合显示半位点结合并在二聚体界面诱导蛋白质动力学。
恶性疟原虫胸苷酸激酶(PfTMK)是生物生长的必需酶,因为它在脱氧胸苷5'-二磷酸(TDP)的从头合成中起着关键作用,脱氧胸苷是5'-二磷酸(TDP)的前体,是DNA复制和修复所需的TTP前体。测量并分析了PfTMK对TMP,dGMP,ADP和ATP的动力学,热力学参数和底物结合特性,并通过稳态动力学以及等温滴定量热法,色氨酸荧光滴定和NMR的组合进行了表征。进行了突变研究,以研究有助于PfTMK也利用dGMP作为底物的独特能力的残基。等温滴定量热法实验表明,dGMP结合表现出独特的半位点结合机制。分子力学计算支持dGMP复合物中空位的封闭。弛豫分散实验表明,在μs-ms时间尺度上,dGMP和酶复合物在二聚体界面上比TMP复合物更动态。在设计基于鸟苷的PfTMK特异性抑制剂时,必须考虑dGMP结合的独特特性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号