Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nitrogen storage regulation by PII protein: lessons learned from taxonomic outliers.
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-01-13 , DOI: 10.1111/febs.15189 Vicente Rubio 1 , Clara Marco-Marín 1 , José Luis Llácer 1
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-01-13 , DOI: 10.1111/febs.15189 Vicente Rubio 1 , Clara Marco-Marín 1 , José Luis Llácer 1
Affiliation
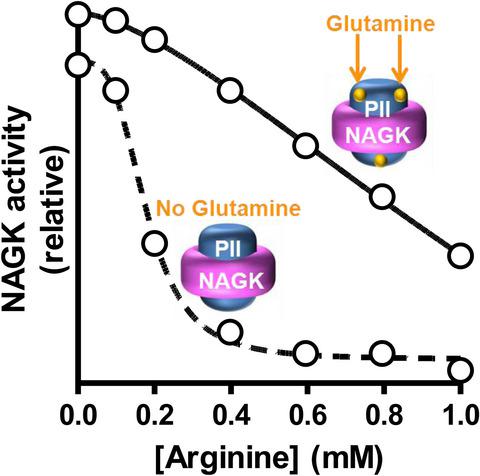
|
The paper 'Interaction of N-acetyl-l-glutamate kinase with the PII signal transducer in the non-photosynthetic alga Polytomella parva: Co-evolution towards a hetero-oligomeric enzyme' by Selim et al. highlights how the study of a true taxonomic oddity, the heterotrophic unicellular alga P. parva, has been instrumental in uncovering the large potential for adaptive variation in the signaling complex of PII with the enzyme N-acetylglutamate kinase (NAGK). This complex modifies the regulatory properties of NAGK, allowing nitrogen stockpiling as arginine. In P. parva, a stable PII-NAGK complex is formed which lacks regulation by canonical PII effectors but which exhibits novel adaptive responses to nitrogen abundance mediated by glutamine, a neo-effector of PII proteins of photosynthetic eukaryotes.
中文翻译:

PII蛋白对氮存储的调控:从分类异常值中吸取的教训。
Selim等人的论文“ N-乙酰基-1-谷氨酸激酶与PII信号传感器在非光合作用藻中的相互作用:共同进化为异寡聚酶”。强调了对真正的分类学奇异性(即异养单细胞藻类P. parva)的研究如何有助于发现PII信号复合物与N-乙酰谷氨酸激酶(NAGK)的适应性变异的巨大潜力。该复合物改变了NAGK的调节特性,允许氮作为精氨酸储存。在P. parva中,形成了稳定的PII-NAGK复合物,该复合物缺乏规范的PII效应子的调控,但对谷氨酰胺(光合作用的真核生物的PII蛋白的新效应子)介导的氮丰度表现出新的适应性反应。
更新日期:2020-02-03
中文翻译:

PII蛋白对氮存储的调控:从分类异常值中吸取的教训。
Selim等人的论文“ N-乙酰基-1-谷氨酸激酶与PII信号传感器在非光合作用藻中的相互作用:共同进化为异寡聚酶”。强调了对真正的分类学奇异性(即异养单细胞藻类P. parva)的研究如何有助于发现PII信号复合物与N-乙酰谷氨酸激酶(NAGK)的适应性变异的巨大潜力。该复合物改变了NAGK的调节特性,允许氮作为精氨酸储存。在P. parva中,形成了稳定的PII-NAGK复合物,该复合物缺乏规范的PII效应子的调控,但对谷氨酰胺(光合作用的真核生物的PII蛋白的新效应子)介导的氮丰度表现出新的适应性反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号