当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Anion binding and fluoride ion induced conformational changes in bisurea receptors
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2020/01/14 , DOI: 10.1039/c9nj05785d Xi Shu 1, 2, 3, 4 , Yu Fan 1, 2, 3, 4 , Shoujian Li 1, 2, 3, 4 , Yongdong Jin 1, 2, 3, 4 , Chuanqin Xia 1, 2, 3, 4 , Chao Huang 1, 2, 3, 4
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2020/01/14 , DOI: 10.1039/c9nj05785d Xi Shu 1, 2, 3, 4 , Yu Fan 1, 2, 3, 4 , Shoujian Li 1, 2, 3, 4 , Yongdong Jin 1, 2, 3, 4 , Chuanqin Xia 1, 2, 3, 4 , Chao Huang 1, 2, 3, 4
Affiliation
|
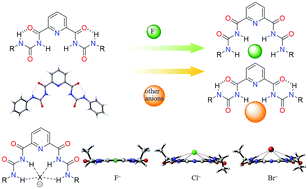
Two types of bisurea receptors, containing either 2,6-substituted phenyl or 2,6-substituted pyridine, are prepared, and their anion binding properties are investigated. Compared with the phenyl bisurea receptors, the pyridine bisurea receptors can be more easily converted to a cis–cis conformation from a trans–trans conformation, providing a cavity that more closely matches the volume of a fluoride ion and increasing the number of NH sites bound to the fluoride ion. As a result, the pyridine bisurea in cis–cis conformation shows stronger affinity and higher selectivity to fluoride ions, which is supported by crystal structure analysis and NMR titration experiments. Through DFT calculations, a mechanism of fluoride ion induced conformational changes of pyridine bisurea receptors is proposed, and the energy barriers of conformational changes for both types of receptors are determined.
中文翻译:

阴离子结合和氟离子诱导的Bisurea受体构象变化
制备了两种含有2,6-取代的苯基或2,6-取代的吡啶的Bisurea受体,并研究了它们的阴离子结合特性。与苯基Bisurea受体相比,吡啶Bisurea受体可以更容易地从反式-反式构象转变为顺式-顺式构象,从而提供一个与氟离子的体积更紧密匹配并增加结合的NH位点的空腔氟离子。结果,吡啶的双氨氮处于顺式–顺式构象表明对氟离子具有更强的亲和力和更高的选择性,这由晶体结构分析和NMR滴定实验证实。通过DFT计算,提出了氟离子诱导吡啶双surea受体构象变化的机理,并确定了两种受体构象变化的能垒。
更新日期:2020-02-10
中文翻译:

阴离子结合和氟离子诱导的Bisurea受体构象变化
制备了两种含有2,6-取代的苯基或2,6-取代的吡啶的Bisurea受体,并研究了它们的阴离子结合特性。与苯基Bisurea受体相比,吡啶Bisurea受体可以更容易地从反式-反式构象转变为顺式-顺式构象,从而提供一个与氟离子的体积更紧密匹配并增加结合的NH位点的空腔氟离子。结果,吡啶的双氨氮处于顺式–顺式构象表明对氟离子具有更强的亲和力和更高的选择性,这由晶体结构分析和NMR滴定实验证实。通过DFT计算,提出了氟离子诱导吡啶双surea受体构象变化的机理,并确定了两种受体构象变化的能垒。



























 京公网安备 11010802027423号
京公网安备 11010802027423号