当前位置:
X-MOL 学术
›
J. Antibiot.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of water-soluble prodrugs of 5-modified 2'-deoxyuridines and their antibacterial activity.
The Journal of Antibiotics ( IF 3.3 ) Pub Date : 2020-01-14 , DOI: 10.1038/s41429-019-0273-x Sergey D Negrya 1 , Maxim V Jasko 1 , Pavel N Solyev 1 , Inna L Karpenko 1 , Olga V Efremenkova 2 , Byazilya F Vasilyeva 2 , Irina G Sumarukova 2 , Sergey N Kochetkov 1 , Liudmila A Alexandrova 1
The Journal of Antibiotics ( IF 3.3 ) Pub Date : 2020-01-14 , DOI: 10.1038/s41429-019-0273-x Sergey D Negrya 1 , Maxim V Jasko 1 , Pavel N Solyev 1 , Inna L Karpenko 1 , Olga V Efremenkova 2 , Byazilya F Vasilyeva 2 , Irina G Sumarukova 2 , Sergey N Kochetkov 1 , Liudmila A Alexandrova 1
Affiliation
|
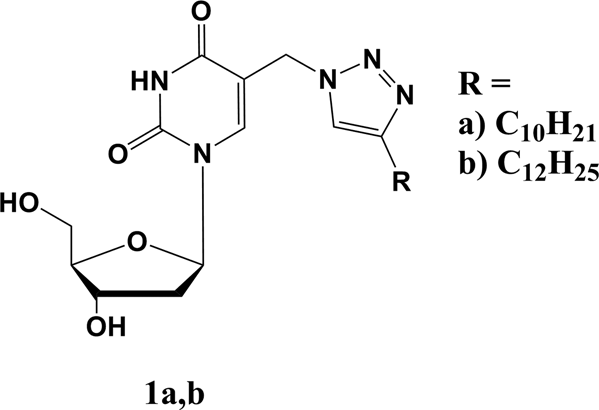
Recently we have synthesized a set of pyrimidine nucleoside derivatives bearing extended alkyltriazolylmethyl substituents at position 5 of the nucleic base, and showed their significant activity against Mycobacterium tuberculosis virulent laboratory strain H37Rv as well as drug-resistant MS-115 strain. The presence of a lengthy hydrophobic substituent leads to the reduction of nucleoside water solubility making their antibacterial activity troublesome to study. A series of water-soluble forms of 5-modified 2'-deoxyuridines 4a-c and 8a-c were synthesized. They appeared at least two orders more soluble compared with the parent compounds 1a and 1b. Their half-hydrolysis time was 5-12 h, which can be considered optimal for prodrugs used in clinics. Obtained compounds showed moderate activity (MIC 48-95 µg·ml-1) against some Gram-positive bacteria including resistant strains of Staphylococcus aureus and Mycobacterium smegmatis and were low cytotoxic for human cell lines (CD50 >> 100 µg·ml-1).
中文翻译:

5-修饰的2'-脱氧尿苷的水溶性前药的合成及其抗菌活性。
最近,我们合成了一组嘧啶核苷衍生物,它们在核酸碱基的5位带有扩展的烷基三唑基甲基取代基,并显示出它们对结核分枝杆菌有毒力的实验室菌株H37Rv和抗药性MS-115菌株的显着活性。较长的疏水取代基的存在导致核苷水溶性降低,从而使其抗菌活性难以研究。合成了一系列水溶性的5-修饰的2'-脱氧尿苷4a-c和8a-c。与母体化合物1a和1b相比,它们的溶解度至少高出两个数量级。它们的半水解时间为5-12小时,对于临床前药而言,这被认为是最佳的。
更新日期:2020-01-14
中文翻译:

5-修饰的2'-脱氧尿苷的水溶性前药的合成及其抗菌活性。
最近,我们合成了一组嘧啶核苷衍生物,它们在核酸碱基的5位带有扩展的烷基三唑基甲基取代基,并显示出它们对结核分枝杆菌有毒力的实验室菌株H37Rv和抗药性MS-115菌株的显着活性。较长的疏水取代基的存在导致核苷水溶性降低,从而使其抗菌活性难以研究。合成了一系列水溶性的5-修饰的2'-脱氧尿苷4a-c和8a-c。与母体化合物1a和1b相比,它们的溶解度至少高出两个数量级。它们的半水解时间为5-12小时,对于临床前药而言,这被认为是最佳的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号