当前位置:
X-MOL 学术
›
PLOS Genet.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Loss of androgen signaling in mesenchymal sonic hedgehog responsive cells diminishes prostate development, growth, and regeneration.
PLOS Genetics ( IF 4.5 ) Pub Date : 2020-01-13 , DOI: 10.1371/journal.pgen.1008588 Vien Le 1 , Yongfeng He 1 , Joseph Aldahl 1 , Erika Hooker 1 , Eun-Jeong Yu 1 , Adam Olson 1 , Won Kyung Kim 1 , Dong-Hoon Lee 1 , Monica Wong 1 , Ruoyu Sheng 1 , Jiaqi Mi 1 , Joseph Geradts 2 , Gerald R Cunha 3 , Zijie Sun 1
PLOS Genetics ( IF 4.5 ) Pub Date : 2020-01-13 , DOI: 10.1371/journal.pgen.1008588 Vien Le 1 , Yongfeng He 1 , Joseph Aldahl 1 , Erika Hooker 1 , Eun-Jeong Yu 1 , Adam Olson 1 , Won Kyung Kim 1 , Dong-Hoon Lee 1 , Monica Wong 1 , Ruoyu Sheng 1 , Jiaqi Mi 1 , Joseph Geradts 2 , Gerald R Cunha 3 , Zijie Sun 1
Affiliation
|
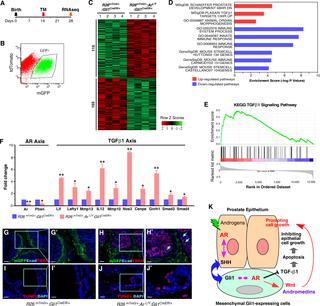
Prostate embryonic development, pubertal and adult growth, maintenance, and regeneration are regulated through androgen signaling-mediated mesenchymal-epithelial interactions. Specifically, the essential role of mesenchymal androgen signaling in the development of prostate epithelium has been observed for over 30 years. However, the identity of the mesenchymal cells responsible for this paracrine regulation and related mechanisms are still unknown. Here, we provide the first demonstration of an indispensable role of the androgen receptor (AR) in sonic hedgehog (SHH) responsive Gli1-expressing cells, in regulating prostate development, growth, and regeneration. Selective deletion of AR expression in Gli1-expressing cells during embryogenesis disrupts prostatic budding and impairs prostate development and formation. Tissue recombination assays showed that urogenital mesenchyme (UGM) containing AR-deficient mesenchymal Gli1-expressing cells combined with wildtype urogenital epithelium (UGE) failed to develop normal prostate tissue in the presence of androgens, revealing the decisive role of AR in mesenchymal SHH responsive cells in prostate development. Prepubescent deletion of AR expression in Gli1-expressing cells resulted in severe impairment of androgen-induced prostate growth and regeneration. RNA-sequencing analysis showed significant alterations in signaling pathways related to prostate development, stem cells, and organ morphogenesis in AR-deficient Gli1-expressing cells. Among these altered pathways, the transforming growth factor β1 (TGFβ1) pathway was up-regulated in AR-deficient Gli1-expressing cells. We further demonstrated the activation of TGFβ1 signaling in AR-deleted prostatic Gli1-expressing cells, which inhibits prostate epithelium growth through paracrine regulation. These data demonstrate a novel role of the AR in the Gli1-expressing cellular niche for regulating prostatic cell fate, morphogenesis, and renewal, and elucidate the mechanism by which mesenchymal androgen-signaling through SHH-responsive cells elicits the growth and regeneration of prostate epithelium.
中文翻译:

间充质声波刺猬反应细胞中雄激素信号的丧失会减少前列腺的发育、生长和再生。
前列腺胚胎发育、青春期和成人生长、维持和再生通过雄激素信号介导的间充质-上皮相互作用进行调节。具体而言,间充质雄激素信号在前列腺上皮发育中的重要作用已经观察了 30 多年。然而,负责这种旁分泌调节和相关机制的间充质细胞的身份仍然未知。在这里,我们首次证明了雄激素受体 (AR) 在声波刺猬 (SHH) 响应性 Gli1 表达细胞中在调节前列腺发育、生长和再生中不可或缺的作用。胚胎发生过程中 Gli1 表达细胞中 AR 表达的选择性缺失会破坏前列腺的出芽并损害前列腺的发育和形成。组织重组分析表明,含有 AR 缺陷型间充质 Gli1 表达细胞的泌尿生殖间充质 (UGM) 与野生型泌尿生殖上皮 (UGE) 在雄激素存在的情况下未能形成正常的前列腺组织,揭示了 AR 在间充质 SHH 反应细胞中的决定性作用在前列腺发育中。Gli1 表达细胞中 AR 表达的青春期前缺失导致雄激素诱导的前列腺生长和再生的严重损害。RNA 测序分析显示,在表达 AR 的 Gli1 细胞中,与前列腺发育、干细胞和器官形态发生相关的信号通路发生了显着改变。在这些改变的通路中,转化生长因子 β1 (TGFβ1) 通路在 AR 缺陷的 Gli1 表达细胞中被上调。我们进一步证明了 AR 缺失的前列腺 Gli1 表达细胞中 TGFβ1 信号的激活,其通过旁分泌调节抑制前列腺上皮生长。这些数据证明了 AR 在表达 Gli1 的细胞生态位中的新作用,用于调节前列腺细胞的命运、形态发生和更新,并阐明了通过 SHH 响应细胞的间充质雄激素信号传导引发前列腺上皮生长和再生的机制.
更新日期:2020-02-18
中文翻译:

间充质声波刺猬反应细胞中雄激素信号的丧失会减少前列腺的发育、生长和再生。
前列腺胚胎发育、青春期和成人生长、维持和再生通过雄激素信号介导的间充质-上皮相互作用进行调节。具体而言,间充质雄激素信号在前列腺上皮发育中的重要作用已经观察了 30 多年。然而,负责这种旁分泌调节和相关机制的间充质细胞的身份仍然未知。在这里,我们首次证明了雄激素受体 (AR) 在声波刺猬 (SHH) 响应性 Gli1 表达细胞中在调节前列腺发育、生长和再生中不可或缺的作用。胚胎发生过程中 Gli1 表达细胞中 AR 表达的选择性缺失会破坏前列腺的出芽并损害前列腺的发育和形成。组织重组分析表明,含有 AR 缺陷型间充质 Gli1 表达细胞的泌尿生殖间充质 (UGM) 与野生型泌尿生殖上皮 (UGE) 在雄激素存在的情况下未能形成正常的前列腺组织,揭示了 AR 在间充质 SHH 反应细胞中的决定性作用在前列腺发育中。Gli1 表达细胞中 AR 表达的青春期前缺失导致雄激素诱导的前列腺生长和再生的严重损害。RNA 测序分析显示,在表达 AR 的 Gli1 细胞中,与前列腺发育、干细胞和器官形态发生相关的信号通路发生了显着改变。在这些改变的通路中,转化生长因子 β1 (TGFβ1) 通路在 AR 缺陷的 Gli1 表达细胞中被上调。我们进一步证明了 AR 缺失的前列腺 Gli1 表达细胞中 TGFβ1 信号的激活,其通过旁分泌调节抑制前列腺上皮生长。这些数据证明了 AR 在表达 Gli1 的细胞生态位中的新作用,用于调节前列腺细胞的命运、形态发生和更新,并阐明了通过 SHH 响应细胞的间充质雄激素信号传导引发前列腺上皮生长和再生的机制.



























 京公网安备 11010802027423号
京公网安备 11010802027423号