当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Toggling Preassembly with Single-Site Mutation Switches the Cytotoxic Mechanism of Cationic Amphipathic Peptides.
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-01-23 , DOI: 10.1021/acs.jmedchem.9b01458 Xiaolong Chen 1, 2 , Shuangshuang Ji 1, 2 , Ang Li 1, 2 , Hanjie Liu 1, 2 , Hao Fei 1, 2
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-01-23 , DOI: 10.1021/acs.jmedchem.9b01458 Xiaolong Chen 1, 2 , Shuangshuang Ji 1, 2 , Ang Li 1, 2 , Hanjie Liu 1, 2 , Hao Fei 1, 2
Affiliation
|
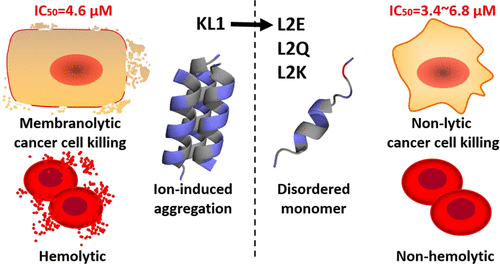
Precise regulation of membrane-active peptide activity is a frontier of research to facilitate its applicational translation. A clear understanding of how a peptide's physicochemical properties determine its mode of action (MOA) will aid the process. Herein, anionic glutamate residue-based scanning was applied to the hydrophobic surface of a self-assembling lysine-rich cationic amphipathic peptide (CAP) KL1. Single-site mutations from leucine to glutamate dramatically changed the MOA of all mutants from membranolytic to nonlytic. An apoptosis-inducing mutant L2E unable to self-assemble under extracellular anions exhibited a different conformational transformation process in the amphiphilic environment than KL1. Further adjustment of the overall positive charge allowed regulation of cytotoxic potency without affecting the MOA determined by the lack of preassembly formation. Compared with KL1, hemolytic toxicities of nonmembranolytic peptides were greatly reduced, with safety indices increased. This work thus provided novel insights into and integrated rationales on the improvement of CAPs for both anticancer activity and safety profile.
中文翻译:

用单位点突变切换预装配可切换阳离子两亲性肽的细胞毒性机制。
膜活性肽活性的精确调节是促进其应用翻译的研究前沿。对肽的理化性质如何决定其作用方式(MOA)的清楚理解将有助于该过程。在此,将基于阴离子谷氨酸残基的扫描应用于自组装的富含赖氨酸的阳离子两亲肽(CAP)KL1的疏水表面。从亮氨酸到谷氨酸的单位点突变极大地改变了所有突变体的MOA,从膜溶解性到非溶解性。在细胞外阴离子下不能自组装的诱导细胞凋亡的突变体L2E在两亲环境中表现出与KL1不同的构象转化过程。总体正电荷的进一步调节允许调节细胞毒性效力,而不会影响由缺乏预装配形成而确定的MOA。与KL1相比,非膜分解肽的溶血毒性大大降低,安全性指标提高。因此,这项工作为改善抗癌活性和安全性方面的CAP提供了新颖的见解,并提供了综合的理论依据。
更新日期:2020-01-23
中文翻译:

用单位点突变切换预装配可切换阳离子两亲性肽的细胞毒性机制。
膜活性肽活性的精确调节是促进其应用翻译的研究前沿。对肽的理化性质如何决定其作用方式(MOA)的清楚理解将有助于该过程。在此,将基于阴离子谷氨酸残基的扫描应用于自组装的富含赖氨酸的阳离子两亲肽(CAP)KL1的疏水表面。从亮氨酸到谷氨酸的单位点突变极大地改变了所有突变体的MOA,从膜溶解性到非溶解性。在细胞外阴离子下不能自组装的诱导细胞凋亡的突变体L2E在两亲环境中表现出与KL1不同的构象转化过程。总体正电荷的进一步调节允许调节细胞毒性效力,而不会影响由缺乏预装配形成而确定的MOA。与KL1相比,非膜分解肽的溶血毒性大大降低,安全性指标提高。因此,这项工作为改善抗癌活性和安全性方面的CAP提供了新颖的见解,并提供了综合的理论依据。


























 京公网安备 11010802027423号
京公网安备 11010802027423号