当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ruthenium(II) Complexes of Heteroditopic N-Heterocyclic Carbene Ligands: Efficient Catalysts for C-N Bond Formation via a Hydrogen-Borrowing Strategy under Solvent-Free Conditions.
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-01-13 , DOI: 10.1021/acs.inorgchem.9b03049 S N R Donthireddy 1 , Praseetha Mathoor Illam 1 , Arnab Rit 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-01-13 , DOI: 10.1021/acs.inorgchem.9b03049 S N R Donthireddy 1 , Praseetha Mathoor Illam 1 , Arnab Rit 1
Affiliation
|
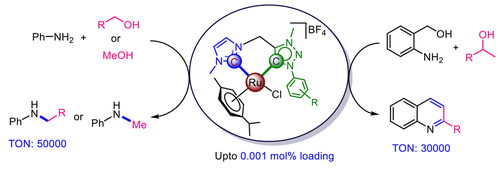
Both imidazol-2-ylidene (ImNHC) and 1,2,3-triazol-5-ylidene (tzNHC) have evolved to be elite groups of N-heterocyclic carbene (NHC) ligands for homogeneous catalysis. To develop efficient ruthenium(II)-based catalysts incorporating these ligands for C-N bond-forming reactions via hydrogen-borrowing methodology, we utilized chelating ligands integrated with ImNHC and mesoionic tzNHC donors connected via a CH2 spacer with a diverse triazole backbone. The synthesized ruthenium(II) complexes 3 are found to be highly efficient for C-N bond formation across a wide range of primary amine and alcohol substrates under solvent-free conditions, and among all of the complexes studied here, catalyst 3a with a mesityl substituent displayed maximum activity. To our delight, catalyst 3a is also effective for the selective mono-N-methylation of various anilines utilizing methanol as a coupling partner, known to be relatively more difficult than other alcohols. Furthermore, complex 3a also delivers various substituted quinolines successfully via the reaction of 2-aminobenzyl alcohol with several secondary alcohols. Importantly, catalyst 3a exhibited the highest activity among the reported ruthenium(II) complexes for both the N-benzylation of aniline [achieving a turnover number (TON) of 50000] and the realization of quinoline 8a by reacting 2-aminobenzyl alcohol with 2-phenylethanol (attaining a TON of 30000).
中文翻译:

异位N-杂环碳配体的钌(II)配合物:在无溶剂条件下通过氢借入策略形成CN键的高效催化剂。
咪唑-2-亚烷基(ImNHC)和1,2,3-三唑-5-亚烷基(tzNHC)均已发展成为用于均相催化的N-杂环卡宾(NHC)配体的精英基团。为了开发通过氢借用方法将这些配体用于CN键形成反应的高效钌(II)基催化剂,我们利用了与ImNHC和通过CH2间隔基与不同的三唑骨架连接的中型tzNHC供体集成的螯合配体。发现合成的钌(II)配合物3在无溶剂条件下能在各种伯胺和醇底物上高效形成CN键,并且在本文研究的所有配合物中,均显示了具有甲磺酰基取代基的催化剂3a最大的活动。令我们高兴的是,催化剂3a对于利用甲醇作为偶合伴侣的各种苯胺的选择性单-N-甲基化也是有效的,众所周知,甲醇作为偶合伴侣比其他醇相对困难。此外,配合物3a还通过2-氨基苄醇与几种仲醇的反应成功地递送了各种取代的喹啉。重要的是,催化剂3a在所报道的钌(II)络合物中对苯胺的N-苄基化[实现营业额(TON)为50000]和通过使2-氨基苄醇与2-氨基反应实现喹啉8a表现出最高的活性。苯乙醇(TON达到30000)。配合物3a还可以通过2-氨基苄醇与几种仲醇的反应成功地提供各种取代的喹啉。重要的是,催化剂3a在所报道的钌(II)络合物中对苯胺的N-苄基化[实现营业额(TON)为50000]和通过使2-氨基苄醇与2-氨基反应实现喹啉8a表现出最高的活性。苯乙醇(TON达到30000)。配合物3a还可以通过2-氨基苄醇与几种仲醇的反应成功地提供各种取代的喹啉。重要的是,催化剂3a在所报道的钌(II)络合物中对苯胺的N-苄基化[实现营业额(TON)为50000]和通过使2-氨基苄醇与2-氨基反应实现喹啉8a表现出最高的活性。苯乙醇(TON达到30000)。
更新日期:2020-01-14
中文翻译:

异位N-杂环碳配体的钌(II)配合物:在无溶剂条件下通过氢借入策略形成CN键的高效催化剂。
咪唑-2-亚烷基(ImNHC)和1,2,3-三唑-5-亚烷基(tzNHC)均已发展成为用于均相催化的N-杂环卡宾(NHC)配体的精英基团。为了开发通过氢借用方法将这些配体用于CN键形成反应的高效钌(II)基催化剂,我们利用了与ImNHC和通过CH2间隔基与不同的三唑骨架连接的中型tzNHC供体集成的螯合配体。发现合成的钌(II)配合物3在无溶剂条件下能在各种伯胺和醇底物上高效形成CN键,并且在本文研究的所有配合物中,均显示了具有甲磺酰基取代基的催化剂3a最大的活动。令我们高兴的是,催化剂3a对于利用甲醇作为偶合伴侣的各种苯胺的选择性单-N-甲基化也是有效的,众所周知,甲醇作为偶合伴侣比其他醇相对困难。此外,配合物3a还通过2-氨基苄醇与几种仲醇的反应成功地递送了各种取代的喹啉。重要的是,催化剂3a在所报道的钌(II)络合物中对苯胺的N-苄基化[实现营业额(TON)为50000]和通过使2-氨基苄醇与2-氨基反应实现喹啉8a表现出最高的活性。苯乙醇(TON达到30000)。配合物3a还可以通过2-氨基苄醇与几种仲醇的反应成功地提供各种取代的喹啉。重要的是,催化剂3a在所报道的钌(II)络合物中对苯胺的N-苄基化[实现营业额(TON)为50000]和通过使2-氨基苄醇与2-氨基反应实现喹啉8a表现出最高的活性。苯乙醇(TON达到30000)。配合物3a还可以通过2-氨基苄醇与几种仲醇的反应成功地提供各种取代的喹啉。重要的是,催化剂3a在所报道的钌(II)络合物中对苯胺的N-苄基化[实现营业额(TON)为50000]和通过使2-氨基苄醇与2-氨基反应实现喹啉8a表现出最高的活性。苯乙醇(TON达到30000)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号