当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unexpected Coordination Complexes of the Metal Tris-silylamides M{N(SiMe3)2}3 (M = Ti, V).
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-01-13 , DOI: 10.1021/acs.inorgchem.9b03084 Cary R Stennett 1 , James C Fettinger 1 , Philip P Power 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-01-13 , DOI: 10.1021/acs.inorgchem.9b03084 Cary R Stennett 1 , James C Fettinger 1 , Philip P Power 1
Affiliation
|
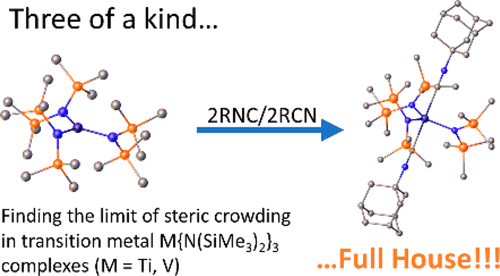
The synthesis, molecular structures, and spectroscopic details of a series of isocyanide and nitrile complexes of the early first-row transition-metal tris(silyl)amides M{N(SiMe3)2}3 (M = Ti, V) are reported. Previously, first-row transition-metal tris(silyl)amides were generally thought to be incapable of forming complexes with Lewis bases due to their excessive steric crowding. However, it is now shown that simple treatment of the base-free trisamides with 2 equiv of an isocyanide or nitrile base at room temperature results in the formation of the trigonal bipyramidal complexes Ti{N(SiMe3)2}3(1-AdNC)2 (1), Ti{N(SiMe3)2}3(CyNC)2 (2), Ti{N(SiMe3)2}3(ButNC)2 (3), Ti{N(SiMe3)2}3(PhCN)2 (4), V{N(SiMe3)2}3(1-AdNC)2 (5), V{N(SiMe3)2}3(CyNC)2 (6), V{N(SiMe3)2}3(ButNC)2 (7), and V{N(SiMe3)2}3(PhCN)2 (8), which incorporate two donor ligands (1-AdNC = 1-adamantyl isocyanide, CyNC = cyclohexyl isocyanide, ButNC = tert-butyl isocyanide, PhCN = benzonitrile). All complexes display a characteristic increase in the frequency of the multiple bonded C-N stretching mode which is observed to be in the range of 2170-2190 cm-1 for the isocyanide complexes 1-3 and 5-7 and at 2250 cm-1 for the nitrile complex 8. This effect was not observed for the titanium nitrile complex 4, suggesting weak binding of the donor to titanium. Paramagnetic 1H NMR studies showed these complexes to have detectable, though extremely broadened, signals attributable to the trimethylsilyl groups of the amide ligands (δ = ca. 2.8 ppm for titanium isocyanide complexes, ca. 4.5-4.7 ppm for vanadium isocyanide complexes). A variable-temperature 1H NMR study showed that in solution these complexes exist as mixtures of the five-coordinate species and a putative four-coordinate species coordinating a single Lewis basic ligand. Electronic spectroscopy indicated that the vanadium complexes 5-8 bind the Lewis bases more strongly than the corresponding titanium complexes, where the spectra of complexes 1-4 are essentially identical to the base-free Ti{N(SiMe3)2}3 at the temperatures and concentrations studied. In contrast to these results, no corresponding complexes were detected for the metal silylamides M{N(SiMe3)2}3 (M = Cr, Mn, Fe, or Co) when treated with the isocyanide or nitrile bases.
中文翻译:

金属三甲硅烷基酰胺M {N(SiMe3)2} 3(M = Ti,V)的意外配位络合物。
报道了第一行过渡金属三(甲硅烷基)酰胺M {N(SiMe3)2} 3(M = Ti,V)的一系列异氰化物和腈配合物的合成,分子结构和光谱学细节。以前,通常认为第一行过渡金属三(甲硅烷基)酰胺由于其过度的空间拥挤而无法与路易斯碱形成络合物。但是,现在表明在室温下用2当量的异氰化物或腈碱对无碱三酰胺进行简单处理会导致形成三角双锥体络合物Ti {N(SiMe3)2} 3(1-AdNC) 2(1),Ti {N(SiMe3)2} 3(CyNC)2(2),Ti {N(SiMe3)2} 3(ButNC)2(3),Ti {N(SiMe3)2} 3(PhCN )2(4),V {N(SiMe3)2} 3(1-AdNC)2(5),V {N(SiMe3)2} 3(CyNC)2(6),V {N(SiMe3)2} 3(ButNC)2(7)和V {N(SiMe3)2} 3(PhCN)2(8),结合了两个供体配体(1-AdNC = 1-金刚烷基异氰化物,CyNC =环己基异氰化物,ButNC =叔丁基异氰化物,PhCN =苄腈)。所有的配合物均表现出多重键合CN拉伸模式的特征性增加,对于异氰酸酯配合物1-3和5-7,观察到的范围为2170-2190 cm-1,对于异氰酸酯配合物为2250 cm-1。腈络合物8。对于钛腈络合物4,未观察到该作用,表明供体与钛的结合较弱。顺磁性1H NMR研究表明,这些配合物具有可检测到的信号(尽管极宽),这归因于酰胺配体的三甲基甲硅烷基(对于异氰酸钛配合物,δ=约2.8 ppm,对于异氰酸钒配合物,约为4.5-4.7 ppm)。可变温度1H NMR研究表明,这些络合物在溶液中以五配位物质和推定的四个Lewis配位体的混合物的形式存在。电子光谱表明,钒配合物5-8比相应的钛配合物更牢固地结合了路易斯碱,其中配合物1-4的光谱在温度下与不含碱的Ti {N(SiMe3)2} 3基本相同。和研究的浓度。与这些结果相反,当用异氰化物或腈碱处理时,未检测到金属甲硅烷基酰胺M {N(SiMe3)2} 3(M = Cr,Mn,Fe或Co)的相应配合物。电子光谱表明,钒配合物5-8比相应的钛配合物更牢固地结合了路易斯碱,其中配合物1-4的光谱在温度下与不含碱的Ti {N(SiMe3)2} 3基本相同。和研究的浓度。与这些结果相反,当用异氰化物或腈碱处理时,未检测到金属甲硅烷基酰胺M {N(SiMe3)2} 3(M = Cr,Mn,Fe或Co)的相应配合物。电子光谱表明,钒配合物5-8比相应的钛配合物更牢固地结合了路易斯碱,其中配合物1-4的光谱在温度下与不含碱的Ti {N(SiMe3)2} 3基本相同。和研究的浓度。与这些结果相反,当用异氰化物或腈碱处理时,未检测到金属甲硅烷基酰胺M {N(SiMe3)2} 3(M = Cr,Mn,Fe或Co)的相应配合物。
更新日期:2020-01-14
中文翻译:

金属三甲硅烷基酰胺M {N(SiMe3)2} 3(M = Ti,V)的意外配位络合物。
报道了第一行过渡金属三(甲硅烷基)酰胺M {N(SiMe3)2} 3(M = Ti,V)的一系列异氰化物和腈配合物的合成,分子结构和光谱学细节。以前,通常认为第一行过渡金属三(甲硅烷基)酰胺由于其过度的空间拥挤而无法与路易斯碱形成络合物。但是,现在表明在室温下用2当量的异氰化物或腈碱对无碱三酰胺进行简单处理会导致形成三角双锥体络合物Ti {N(SiMe3)2} 3(1-AdNC) 2(1),Ti {N(SiMe3)2} 3(CyNC)2(2),Ti {N(SiMe3)2} 3(ButNC)2(3),Ti {N(SiMe3)2} 3(PhCN )2(4),V {N(SiMe3)2} 3(1-AdNC)2(5),V {N(SiMe3)2} 3(CyNC)2(6),V {N(SiMe3)2} 3(ButNC)2(7)和V {N(SiMe3)2} 3(PhCN)2(8),结合了两个供体配体(1-AdNC = 1-金刚烷基异氰化物,CyNC =环己基异氰化物,ButNC =叔丁基异氰化物,PhCN =苄腈)。所有的配合物均表现出多重键合CN拉伸模式的特征性增加,对于异氰酸酯配合物1-3和5-7,观察到的范围为2170-2190 cm-1,对于异氰酸酯配合物为2250 cm-1。腈络合物8。对于钛腈络合物4,未观察到该作用,表明供体与钛的结合较弱。顺磁性1H NMR研究表明,这些配合物具有可检测到的信号(尽管极宽),这归因于酰胺配体的三甲基甲硅烷基(对于异氰酸钛配合物,δ=约2.8 ppm,对于异氰酸钒配合物,约为4.5-4.7 ppm)。可变温度1H NMR研究表明,这些络合物在溶液中以五配位物质和推定的四个Lewis配位体的混合物的形式存在。电子光谱表明,钒配合物5-8比相应的钛配合物更牢固地结合了路易斯碱,其中配合物1-4的光谱在温度下与不含碱的Ti {N(SiMe3)2} 3基本相同。和研究的浓度。与这些结果相反,当用异氰化物或腈碱处理时,未检测到金属甲硅烷基酰胺M {N(SiMe3)2} 3(M = Cr,Mn,Fe或Co)的相应配合物。电子光谱表明,钒配合物5-8比相应的钛配合物更牢固地结合了路易斯碱,其中配合物1-4的光谱在温度下与不含碱的Ti {N(SiMe3)2} 3基本相同。和研究的浓度。与这些结果相反,当用异氰化物或腈碱处理时,未检测到金属甲硅烷基酰胺M {N(SiMe3)2} 3(M = Cr,Mn,Fe或Co)的相应配合物。电子光谱表明,钒配合物5-8比相应的钛配合物更牢固地结合了路易斯碱,其中配合物1-4的光谱在温度下与不含碱的Ti {N(SiMe3)2} 3基本相同。和研究的浓度。与这些结果相反,当用异氰化物或腈碱处理时,未检测到金属甲硅烷基酰胺M {N(SiMe3)2} 3(M = Cr,Mn,Fe或Co)的相应配合物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号