当前位置:
X-MOL 学术
›
Chemistryopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
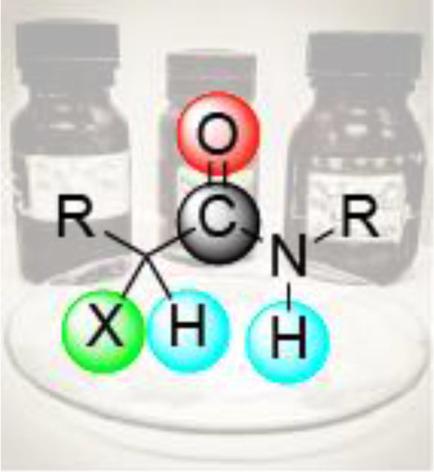
The Fascinating Chemistry of α-Haloamides.
ChemistryOpen ( IF 2.3 ) Pub Date : 2020-01-13 , DOI: 10.1002/open.201900220 Anna Fantinati 1 , Vinicio Zanirato 1 , Paolo Marchetti 1 , Claudio Trapella 1
ChemistryOpen ( IF 2.3 ) Pub Date : 2020-01-13 , DOI: 10.1002/open.201900220 Anna Fantinati 1 , Vinicio Zanirato 1 , Paolo Marchetti 1 , Claudio Trapella 1
Affiliation
|
The aim of this review is to highlight the rich chemistry of α‐haloamides originally mainly used to discover new C−N, C−O and C−S bond forming reactions, and later widely employed in C−C cross‐coupling reactions with C(sp3), C(sp2) and C(sp) coupling partners. Radical‐mediated transformations of α‐haloamides bearing a suitable located unsaturated bond has proven to be a straightforward alternative to access diverse cyclic compounds by means of either radical initiators, transition metal redox catalysis or visible light photoredox catalysis. On the other hand, cycloadditions with α‐halohydroxamate‐based azaoxyallyl cations have garnered significant attention. Moreover, in view of the important role in life and materials science of difluoroalkylated compounds, a wide range of catalysts has been developed for the efficient incorporation of difluoroacetamido moieties into activated as well as unactivated substrates.
中文翻译:

α-卤代酰胺的迷人化学。
本综述的目的是强调α-卤代酰胺丰富的化学性质,最初主要用于发现新的 C−N、C−O 和 C−S 键形成反应,后来广泛应用于与 C 的 C−C 交叉偶联反应(sp 3 )、C(sp 2 ) 和 C(sp) 偶联伙伴。带有合适位置的不饱和键的α-卤代酰胺的自由基介导的转化已被证明是通过自由基引发剂、过渡金属氧化还原催化或可见光光氧化还原催化获得多种环状化合物的直接替代方案。另一方面,与基于α-卤代异羟肟酸酯的氮杂氧基烯丙基阳离子的环加成引起了人们的广泛关注。此外,鉴于二氟烷基化化合物在生命和材料科学中的重要作用,已经开发了多种催化剂用于将二氟乙酰胺部分有效地掺入活化和未活化的底物中。
更新日期:2020-01-13
中文翻译:

α-卤代酰胺的迷人化学。
本综述的目的是强调α-卤代酰胺丰富的化学性质,最初主要用于发现新的 C−N、C−O 和 C−S 键形成反应,后来广泛应用于与 C 的 C−C 交叉偶联反应(sp 3 )、C(sp 2 ) 和 C(sp) 偶联伙伴。带有合适位置的不饱和键的α-卤代酰胺的自由基介导的转化已被证明是通过自由基引发剂、过渡金属氧化还原催化或可见光光氧化还原催化获得多种环状化合物的直接替代方案。另一方面,与基于α-卤代异羟肟酸酯的氮杂氧基烯丙基阳离子的环加成引起了人们的广泛关注。此外,鉴于二氟烷基化化合物在生命和材料科学中的重要作用,已经开发了多种催化剂用于将二氟乙酰胺部分有效地掺入活化和未活化的底物中。


























 京公网安备 11010802027423号
京公网安备 11010802027423号