当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery of Compounds Inhibiting the ADP-Ribosyltransferase Activity of Pertussis Toxin.
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2020-01-13 , DOI: 10.1021/acsinfecdis.9b00412 Yashwanth Ashok 1 , Moona Miettinen 2, 3 , Danilo Kimio Hirabae de Oliveira 1 , Mahlet Z Tamirat 4 , Katja Näreoja 2 , Avlokita Tiwari 2 , Michael O Hottiger 5 , Mark S Johnson 4 , Lari Lehtiö 1 , Arto T Pulliainen 2
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2020-01-13 , DOI: 10.1021/acsinfecdis.9b00412 Yashwanth Ashok 1 , Moona Miettinen 2, 3 , Danilo Kimio Hirabae de Oliveira 1 , Mahlet Z Tamirat 4 , Katja Näreoja 2 , Avlokita Tiwari 2 , Michael O Hottiger 5 , Mark S Johnson 4 , Lari Lehtiö 1 , Arto T Pulliainen 2
Affiliation
|
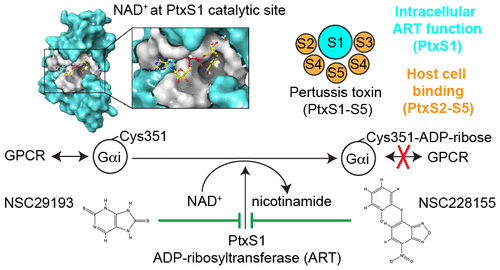
The targeted pathogen-selective approach to drug development holds promise to minimize collateral damage to the beneficial microbiome. The AB5-topology pertussis toxin (PtxS1-S5) is a major virulence factor of Bordetella pertussis, the causative agent of the highly contagious respiratory disease whooping cough. Once internalized into the host cell, PtxS1 ADP-ribosylates α-subunits of the heterotrimeric Gαi-superfamily, thereby disrupting G-protein-coupled receptor signaling. Here, we report the discovery of the first small molecules inhibiting the ADP-ribosyltransferase activity of pertussis toxin. We developed protocols to purify milligram-levels of active recombinant B. pertussis PtxS1 from Escherichia coli and an in vitro high throughput-compatible assay to quantify NAD+ consumption during PtxS1-catalyzed ADP-ribosylation of Gαi. Two inhibitory compounds (NSC228155 and NSC29193) with low micromolar IC50-values (3.0 μM and 6.8 μM) were identified in the in vitro NAD+ consumption assay that also were potent in an independent in vitro assay monitoring conjugation of ADP-ribose to Gαi. Docking and molecular dynamics simulations identified plausible binding poses of NSC228155 and in particular of NSC29193, most likely owing to the rigidity of the latter ligand, at the NAD+-binding pocket of PtxS1. NSC228155 inhibited the pertussis AB5 holotoxin-catalyzed ADP-ribosylation of Gαi in living human cells with a low micromolar IC50-value (2.4 μM). NSC228155 and NSC29193 might prove to be useful hit compounds in targeted B. pertussis-selective drug development.
中文翻译:

发现抑制百日咳毒素ADP-核糖基转移酶活性的化合物。
有针对性的病原体选择性药物开发方法有望将对有益微生物组的附带损害降至最低。AB5拓扑百日咳毒素(PtxS1-S5)是百日咳博德特氏菌的主要毒力因子,百日咳博德特氏菌是百日咳高传染性呼吸道疾病的病原。一旦内化到宿主细胞中,PtxS1 ADP-核糖基化异三聚体Gαi超家族的α亚基,从而破坏G蛋白偶联的受体信号传导。在这里,我们报告发现抑制百日咳毒素的ADP-核糖基转移酶活性的第一个小分子的发现。我们开发了从大肠杆菌中纯化活性重组百日咳博德特氏菌PtxS1毫克级的方案,并进行了体外高通量兼容测定,以量化PtxS1催化Gαi的ADP-核糖基化过程中的NAD +消耗。在体外NAD +消耗试验中鉴定出两种具有低微摩尔IC50值(3.0μM和6.8μM)的抑制性化合物(NSC228155和NSC29193),它们在监测ADP-核糖与Gαi结合的独立体外试验中也很有效。对接和分子动力学模拟在PtxS1的NAD +结合口袋处确定了NSC228155尤其是NSC29193的合理结合姿势,这很可能是由于后者配体的刚性所致。NSC228155以低微摩尔IC50值(2.4μM)抑制了百日咳AB5全息毒素催化Gαi的ADP核糖基化。NSC228155和NSC29193可能被证明是针对百日咳博德特氏菌选择性药物开发的有用命中化合物。在体外NAD +消耗试验中鉴定出8μM),在监测ADP-核糖与Gαi结合的独立体外试验中也很有效。对接和分子动力学模拟在PtxS1的NAD +结合口袋处确定了NSC228155尤其是NSC29193的合理结合姿势,这很可能是由于后者配体的刚性所致。NSC228155以低微摩尔IC50值(2.4μM)抑制了百日咳AB5全息毒素催化Gαi的ADP核糖基化。NSC228155和NSC29193可能被证明是针对百日咳博德特氏菌选择性药物开发的有用命中化合物。在体外NAD +消耗试验中鉴定出8μM),在监测ADP-核糖与Gαi结合的独立体外试验中也很有效。对接和分子动力学模拟在PtxS1的NAD +结合口袋处确定了NSC228155尤其是NSC29193的合理结合姿势,这很可能是由于后者配体的刚性所致。NSC228155以低微摩尔IC50值(2.4μM)抑制了百日咳AB5全息毒素催化Gαi的ADP核糖基化。NSC228155和NSC29193可能被证明是针对百日咳博德特氏菌选择性药物开发的有用命中化合物。对接和分子动力学模拟在PtxS1的NAD +结合口袋处确定了NSC228155尤其是NSC29193的合理结合姿势,这很可能是由于后者配体的刚性所致。NSC228155以低微摩尔IC50值(2.4μM)抑制了百日咳AB5全息毒素催化Gαi的ADP核糖基化。NSC228155和NSC29193可能被证明是针对百日咳博德特氏菌选择性药物开发的有用命中化合物。对接和分子动力学模拟在PtxS1的NAD +结合口袋处确定了NSC228155尤其是NSC29193的合理结合姿势,这很可能是由于后者配体的刚性所致。NSC228155以低微摩尔IC50值(2.4μM)抑制了百日咳AB5全息毒素催化Gαi的ADP核糖基化。NSC228155和NSC29193可能被证明是针对百日咳博德特氏菌选择性药物开发的有用命中化合物。
更新日期:2020-01-03
中文翻译:

发现抑制百日咳毒素ADP-核糖基转移酶活性的化合物。
有针对性的病原体选择性药物开发方法有望将对有益微生物组的附带损害降至最低。AB5拓扑百日咳毒素(PtxS1-S5)是百日咳博德特氏菌的主要毒力因子,百日咳博德特氏菌是百日咳高传染性呼吸道疾病的病原。一旦内化到宿主细胞中,PtxS1 ADP-核糖基化异三聚体Gαi超家族的α亚基,从而破坏G蛋白偶联的受体信号传导。在这里,我们报告发现抑制百日咳毒素的ADP-核糖基转移酶活性的第一个小分子的发现。我们开发了从大肠杆菌中纯化活性重组百日咳博德特氏菌PtxS1毫克级的方案,并进行了体外高通量兼容测定,以量化PtxS1催化Gαi的ADP-核糖基化过程中的NAD +消耗。在体外NAD +消耗试验中鉴定出两种具有低微摩尔IC50值(3.0μM和6.8μM)的抑制性化合物(NSC228155和NSC29193),它们在监测ADP-核糖与Gαi结合的独立体外试验中也很有效。对接和分子动力学模拟在PtxS1的NAD +结合口袋处确定了NSC228155尤其是NSC29193的合理结合姿势,这很可能是由于后者配体的刚性所致。NSC228155以低微摩尔IC50值(2.4μM)抑制了百日咳AB5全息毒素催化Gαi的ADP核糖基化。NSC228155和NSC29193可能被证明是针对百日咳博德特氏菌选择性药物开发的有用命中化合物。在体外NAD +消耗试验中鉴定出8μM),在监测ADP-核糖与Gαi结合的独立体外试验中也很有效。对接和分子动力学模拟在PtxS1的NAD +结合口袋处确定了NSC228155尤其是NSC29193的合理结合姿势,这很可能是由于后者配体的刚性所致。NSC228155以低微摩尔IC50值(2.4μM)抑制了百日咳AB5全息毒素催化Gαi的ADP核糖基化。NSC228155和NSC29193可能被证明是针对百日咳博德特氏菌选择性药物开发的有用命中化合物。在体外NAD +消耗试验中鉴定出8μM),在监测ADP-核糖与Gαi结合的独立体外试验中也很有效。对接和分子动力学模拟在PtxS1的NAD +结合口袋处确定了NSC228155尤其是NSC29193的合理结合姿势,这很可能是由于后者配体的刚性所致。NSC228155以低微摩尔IC50值(2.4μM)抑制了百日咳AB5全息毒素催化Gαi的ADP核糖基化。NSC228155和NSC29193可能被证明是针对百日咳博德特氏菌选择性药物开发的有用命中化合物。对接和分子动力学模拟在PtxS1的NAD +结合口袋处确定了NSC228155尤其是NSC29193的合理结合姿势,这很可能是由于后者配体的刚性所致。NSC228155以低微摩尔IC50值(2.4μM)抑制了百日咳AB5全息毒素催化Gαi的ADP核糖基化。NSC228155和NSC29193可能被证明是针对百日咳博德特氏菌选择性药物开发的有用命中化合物。对接和分子动力学模拟在PtxS1的NAD +结合口袋处确定了NSC228155尤其是NSC29193的合理结合姿势,这很可能是由于后者配体的刚性所致。NSC228155以低微摩尔IC50值(2.4μM)抑制了百日咳AB5全息毒素催化Gαi的ADP核糖基化。NSC228155和NSC29193可能被证明是针对百日咳博德特氏菌选择性药物开发的有用命中化合物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号