当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereoselective synthesis of amino-substituted cyclopentafullerenes promoted by magnesium perchlorate/ferric perchlorate.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-01-13 , DOI: 10.1039/c9ob02248a Wan Ma 1 , Kun Wang 1 , Cheng Huang 1 , Hui-Juan Wang 2 , Fa-Bao Li 1 , Rui Sun 1 , Li Liu 1 , Chao-Yang Liu 2 , Abdullah M Asiri 3
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-01-13 , DOI: 10.1039/c9ob02248a Wan Ma 1 , Kun Wang 1 , Cheng Huang 1 , Hui-Juan Wang 2 , Fa-Bao Li 1 , Rui Sun 1 , Li Liu 1 , Chao-Yang Liu 2 , Abdullah M Asiri 3
Affiliation
|
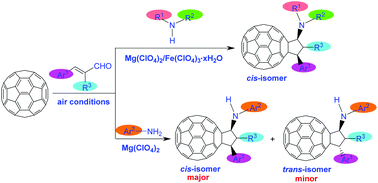
A facile one-step reaction of [60]fullerene with cinnamaldehydes and amines promoted by magnesium perchlorate/ferric perchlorate under air conditions afforded a series of rare amino-substituted cyclopentafullerenes in moderate to good yields. Stereoselectivity was readily achieved. Secondary amines exclusively produced N,N-disubstituted cyclopentafullerenes as cis isomers, while arylamines gave N-monosubstituted cyclopentafullerenes with a preference of cis isomers as major products. N-Monosubstituted cyclopentafullerenes could be further converted into other scarce cyclopentafullerenes in the presence of acid chloride or paraformaldehyde. A possible reaction pathway was proposed to elucidate the formation of amino-substituted cyclopentafullerenes.
中文翻译:

高氯酸镁/高氯酸铁促进的氨基取代的环戊富勒烯的立体选择性合成。
在空气条件下,高氯酸镁/高氯酸铁促进的[60]富勒烯与肉桂醛和胺的简便一步反应,以中等至良好的收率得到了一系列稀有的氨基取代的环戊富勒烯。立体选择性很容易实现。仲胺专门生产N,N-二取代的环戊富勒烯为顺式异构体,而芳基胺则提供N-单取代的环戊富勒烯,以顺式异构体为主要产物。在酰氯或低聚甲醛的存在下,N-单取代的环戊富勒烯可以进一步转化为其他稀缺的环戊富勒烯。提出了可能的反应途径来阐明氨基取代的环戊富勒烯的形成。
更新日期:2020-02-13
中文翻译:

高氯酸镁/高氯酸铁促进的氨基取代的环戊富勒烯的立体选择性合成。
在空气条件下,高氯酸镁/高氯酸铁促进的[60]富勒烯与肉桂醛和胺的简便一步反应,以中等至良好的收率得到了一系列稀有的氨基取代的环戊富勒烯。立体选择性很容易实现。仲胺专门生产N,N-二取代的环戊富勒烯为顺式异构体,而芳基胺则提供N-单取代的环戊富勒烯,以顺式异构体为主要产物。在酰氯或低聚甲醛的存在下,N-单取代的环戊富勒烯可以进一步转化为其他稀缺的环戊富勒烯。提出了可能的反应途径来阐明氨基取代的环戊富勒烯的形成。

























 京公网安备 11010802027423号
京公网安备 11010802027423号