当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Routes of iron entry into, and exit from, the catalytic ferroxidase sites of the prokaryotic ferritin SynFtn.
Dalton Transactions ( IF 4 ) Pub Date : 2020-01-13 , DOI: 10.1039/c9dt03570b Justin M Bradley 1 , Jacob Pullin , Geoffrey R Moore , Dimitri A Svistunenko , Andrew M Hemmings , Nick E Le Brun
Dalton Transactions ( IF 4 ) Pub Date : 2020-01-13 , DOI: 10.1039/c9dt03570b Justin M Bradley 1 , Jacob Pullin , Geoffrey R Moore , Dimitri A Svistunenko , Andrew M Hemmings , Nick E Le Brun
Affiliation
|
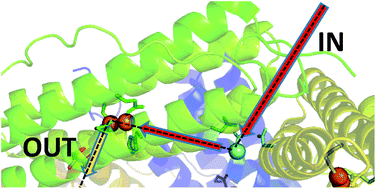
Ferritins are multimers comprised of 4 α-helical bundle monomers that co-assemble to form protein shells surrounding an approximately spherical internal cavity. The assembled multimers acquire Fe2+ from their surroundings by utilising channels that penetrate the protein for the transportation of iron to diiron catalytic centres buried within the monomeric units. Here oxidation of the substrate to Fe3+ is coupled to the reduction of O2 and/or peroxide to yield the precursor to a ferric oxy hydroxide mineral that is stored within the internal cavity. The rhombic dodecahedral quaternary structure results in channels of 4-fold and 3-fold symmetry, located at the vertices, which are common to all 24mer-ferritins. Ferritins isolated from higher eukaryotes have been demonstrated to take up Fe2+via the 3-fold channels. One of the defining features of ferritins isolated from prokaryotes is the presence of a further 24 channels, the B-channels, and these are thought to play an important role in Fe2+ uptake in this sub-family. SynFtn is an unusual ferritin isolated from the marine cyanobacterium Synechococcus CC9311. The reported structure of SynFtn derived from Fe2+ soaked crystals revealed the presence of a fully hydrated Fe2+ associated with three aspartate residues (Asp137 from each of the three symmetry related subunits) within each three-fold channel, suggesting that it might be the route for Fe2+ entry. Here, we present structural and spectro-kinetic data on two variants of SynFtn, D137A and E62A, designed to assess this possibility. Glu62 is equivalent to residues demonstrated to be important in the transfer of iron from the inner exit of the 3-fold channel to the catalytic centre in animal ferritins. As expected replacing Asp137 with a non-coordinating residue eliminated rapid iron oxidation by SynFtn. In contrast the rate of mineral core formation was severely impaired whilst the rate of iron transit into the catalytic centre was largely unaffected upon introducing a non-coordinating residue in place of Glu62 suggesting a role for this residue in release of the oxidised product. The identification of these two residues in SynFtn maps out major routes for Fe2+ entry to, and exit from, the catalytic ferroxidase centres.
中文翻译:

铁进入原核铁蛋白SynFtn的催化铁氧化酶位点和从中出来的途径。
铁蛋白是由4个α-螺旋束单体组成的多聚体,它们共组装形成围绕大约球形内部空腔的蛋白质壳。组装的多聚体通过利用可穿透蛋白质的通道将铁运输到掩埋在单体单元中的二铁催化中心而从周围环境中获取Fe2 +。此处,将底物氧化为Fe3 +与O2和/或过氧化物的还原反应相关联,以将前体生成储存在内部空腔中的氢氧化铁三元铁矿物质。菱形十二面体的四级结构导致对称的4倍和3倍通道位于所有24mer-铁蛋白共有的顶点。从高等真核生物中分离出的铁蛋白已被证明通过3倍通道吸收Fe2 +。从原核生物中分离出的铁蛋白的主要特征之一是还存在24个通道,即B通道,这些通道被认为在该亚家族的Fe2 +吸收中起重要作用。SynFtn是从海洋蓝藻Synechococcus CC9311中分离出来的一种不寻常的铁蛋白。已报道的Fe2 +浸泡晶体的SynFtn结构表明,在每个三折通道中存在与三个天冬氨酸残基(来自三个对称相关亚基中的每一个的Asp137)相关的完全水合的Fe2 +,这表明这可能是Fe2 +的途径条目。在这里,我们介绍了SynFtn的两个变体D137A和E62A的结构和光谱动力学数据,旨在评估这种可能性。Glu62等同于被证明在动物铁蛋白中将铁从3倍通道的内部出口转移到催化中心方面很重要的残基。如预期的那样,用非配位残基替代Asp137消除了SynFtn引起的铁快速氧化。相反,在引入非配位残基代替Glu62时,严重影响了矿物芯形成速率,而铁向催化中心的转运速率基本不受影响,表明该残基在释放氧化产物中起作用。SynFtn中这两个残基的鉴定为Fe2 +进入和离开催化铁氧化酶中心的主要路线图。相反,在引入非配位残基代替Glu62时,严重影响了矿物芯形成速率,而铁向催化中心的转运速率基本不受影响,表明该残基在释放氧化产物中起作用。SynFtn中这两个残基的鉴定为Fe2 +进入和离开催化铁氧化酶中心的主要路线图。相反,在引入非配位残基代替Glu62时,严重影响了矿物芯形成速率,而铁向催化中心的转运速率基本不受影响,表明该残基在释放氧化产物中起作用。SynFtn中这两个残基的鉴定为Fe2 +进入和离开催化铁氧化酶中心的主要路线图。
更新日期:2020-02-10
中文翻译:

铁进入原核铁蛋白SynFtn的催化铁氧化酶位点和从中出来的途径。
铁蛋白是由4个α-螺旋束单体组成的多聚体,它们共组装形成围绕大约球形内部空腔的蛋白质壳。组装的多聚体通过利用可穿透蛋白质的通道将铁运输到掩埋在单体单元中的二铁催化中心而从周围环境中获取Fe2 +。此处,将底物氧化为Fe3 +与O2和/或过氧化物的还原反应相关联,以将前体生成储存在内部空腔中的氢氧化铁三元铁矿物质。菱形十二面体的四级结构导致对称的4倍和3倍通道位于所有24mer-铁蛋白共有的顶点。从高等真核生物中分离出的铁蛋白已被证明通过3倍通道吸收Fe2 +。从原核生物中分离出的铁蛋白的主要特征之一是还存在24个通道,即B通道,这些通道被认为在该亚家族的Fe2 +吸收中起重要作用。SynFtn是从海洋蓝藻Synechococcus CC9311中分离出来的一种不寻常的铁蛋白。已报道的Fe2 +浸泡晶体的SynFtn结构表明,在每个三折通道中存在与三个天冬氨酸残基(来自三个对称相关亚基中的每一个的Asp137)相关的完全水合的Fe2 +,这表明这可能是Fe2 +的途径条目。在这里,我们介绍了SynFtn的两个变体D137A和E62A的结构和光谱动力学数据,旨在评估这种可能性。Glu62等同于被证明在动物铁蛋白中将铁从3倍通道的内部出口转移到催化中心方面很重要的残基。如预期的那样,用非配位残基替代Asp137消除了SynFtn引起的铁快速氧化。相反,在引入非配位残基代替Glu62时,严重影响了矿物芯形成速率,而铁向催化中心的转运速率基本不受影响,表明该残基在释放氧化产物中起作用。SynFtn中这两个残基的鉴定为Fe2 +进入和离开催化铁氧化酶中心的主要路线图。相反,在引入非配位残基代替Glu62时,严重影响了矿物芯形成速率,而铁向催化中心的转运速率基本不受影响,表明该残基在释放氧化产物中起作用。SynFtn中这两个残基的鉴定为Fe2 +进入和离开催化铁氧化酶中心的主要路线图。相反,在引入非配位残基代替Glu62时,严重影响了矿物芯形成速率,而铁向催化中心的转运速率基本不受影响,表明该残基在释放氧化产物中起作用。SynFtn中这两个残基的鉴定为Fe2 +进入和离开催化铁氧化酶中心的主要路线图。


























 京公网安备 11010802027423号
京公网安备 11010802027423号