Communications Physics ( IF 5.5 ) Pub Date : 2020-01-13 , DOI: 10.1038/s42005-019-0270-1 Mohamed Ali al-Badri , Edward Linscott , Antoine Georges , Daniel J. Cole , Cédric Weber
|
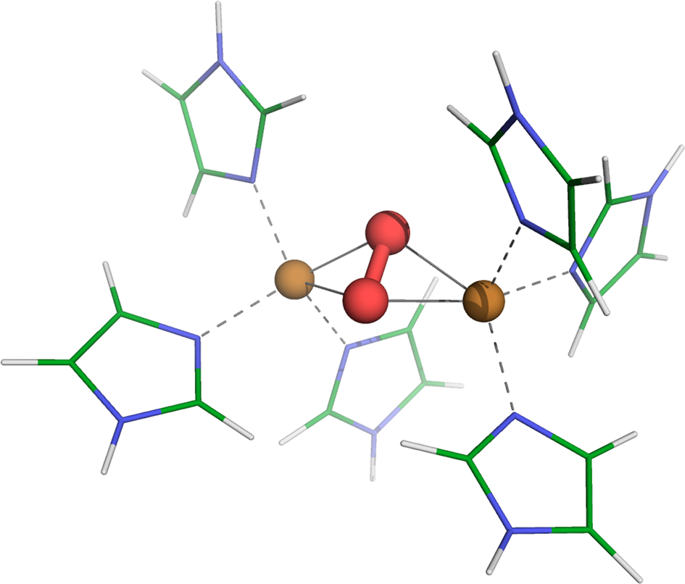
The hemocyanin protein binds and transports molecular oxygen via two copper atoms at its core. The singlet state of the \({{\rm{Cu}}}_{2}{{\rm{O}}}_{2}\) core is thought to be stabilised by a superexchange pathway, but detailed in situ computational analysis is complicated by the multi-reference character of the electronic ground state. Here, electronic correlation effects in the functional site of hemocyanin are investigated using a novel approach, treating the localised copper 3d electrons with cluster dynamical mean field theory. This enables us to account for dynamical and multi-reference quantum mechanics, capturing valence and spin fluctuations of the 3d electrons. Our approach explains the stabilisation of the experimentally observed di-Cu singlet for the butterflied \({{\rm{Cu}}}_{2}{{\rm{O}}}_{2}\) core, with localised charge and incoherent scattering processes across the oxo-bridge that prevent long-lived charge excitations. This suggests that the magnetic structure of hemocyanin is largely influenced by the many-body corrections.
中文翻译:

原型双铜氧桥中的超交换机理和量子多体激发
血色素蛋白通过其核心的两个铜原子结合并转运分子氧。的单重态\({{\ RM {的Cu}}} _ {2} {{\ RM {ö}}} _ {2} \)芯被认为是由一个超交换途径来稳定,但在原位详述电子基态的多参考特征使计算分析变得复杂。在这里,使用一种新颖的方法研究血蓝蛋白功能位点的电子相关效应,并用簇动力学平均场理论处理局部3d铜电子。这使我们能够考虑动力学和多参考量子力学,捕获3d电子的化合价和自旋涨落。我们的方法解释了实验观察到的双铜单峰的稳定\({{\ rm {Cu}}} _ {2} {{\ rm {O}}} _ {2} \)核,在氧桥上具有局部电荷和不相干的散射过程,可防止长寿命电荷激发。这表明血蓝蛋白的磁性结构在很大程度上受到多体校正的影响。

























 京公网安备 11010802027423号
京公网安备 11010802027423号