当前位置:
X-MOL 学术
›
J. Colloid Interface Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The impact of size and surface ligand of gold nanorods on liver cancer accumulation and photothermal therapy in the second near-infrared window.
Journal of Colloid and Interface Science ( IF 9.9 ) Pub Date : 2020-01-13 , DOI: 10.1016/j.jcis.2020.01.026 Huang Yang 1 , Hongpeng He 2 , Zongrui Tong 1 , Haibing Xia 2 , Zhengwei Mao 1 , Changyou Gao 1
Journal of Colloid and Interface Science ( IF 9.9 ) Pub Date : 2020-01-13 , DOI: 10.1016/j.jcis.2020.01.026 Huang Yang 1 , Hongpeng He 2 , Zongrui Tong 1 , Haibing Xia 2 , Zhengwei Mao 1 , Changyou Gao 1
Affiliation
|
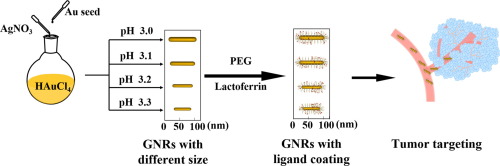
Gold nanorods (GNRs) with longitudinal surface plasmon resonance (LSPR) peaks in second near-infrared (NIR-II) window have attracted a great amount of attention as photothermal transducer because of their inherently excellent photothermal transition efficiency, high biocompatibility and versatile surface functionalization. One key question for the application of these GNRs against tumors in vivo is which size/shape and surface ligand conjugation are promising for circulation and tumor targeting. In this study, we prepared a series of gold nanorods (GNRs) of similar aspect ratio and LSPR peaks, and thus similar photothermal transfer efficiency under irradiation of 980 nm laser, but with tunable size in width and length. The obtained GNRs were subjected to surface modification with PEG and tumor targeting ligand lactoferrin. With these tailor-designed GNRs in hand, we have the chance to study the impact of dimension and surface property of the GNRs on their internalization via tumor cells, photothermal cytotoxicity in vitro, blood circulation and tissue distribution pattern in vivo. As a result, the GNRs with medium size (70 nm in length and 11.5 nm in width) and surface PEG/LF modification (GNR70@PEG-LF) exhibit the fastest cell internalization via HepG2 cells and best photothermal outcome in vitro. The GNR70@PEG-LF also display long circulation time and the highest tumor accumulation in vivo, due to the synergetic effect of surface coating and dimension. Finally, tumor ablation ability of the GNRs under irradiation of 980 nm light were validated on mice xenograft model, suggesting their potential photothermal therapy against cancer in NIR-II window.
中文翻译:

在第二个近红外窗口中,金纳米棒的大小和表面配体对肝癌积累和光热疗法的影响。
在第二近红外(NIR-II)窗口中具有纵向表面等离子体共振(LSPR)峰的金纳米棒(GNR)作为光热换能器吸引了众多关注,因为它们固有的优异的光热转换效率,高生物相容性和通用的表面功能化。这些GNRs在体内对肿瘤的应用的一个关键问题是哪种大小/形状和表面配体缀合有望用于循环和靶向肿瘤。在这项研究中,我们制备了一系列长宽比和LSPR峰相似的金纳米棒(GNR),因此在980 nm激光辐照下具有相似的光热传递效率,但宽度和长度均可调。将获得的GNR用PEG和肿瘤靶向配体乳铁蛋白进行表面修饰。有了这些量身定制的GNR,我们就有机会通过肿瘤细胞,体外光热细胞毒性,体内血液循环和组织分布模式研究GNR的尺寸和表面特性对其内在化的影响。结果,中等大小(长度为70 nm,宽度为11.5 nm)和表面PEG / LF修饰(GNR70 @ PEG-LF)的GNR在体外通过HepG2细胞表现出最快的细胞内在化和最佳光热结果。由于表面涂层和尺寸的协同作用,GNR70 @ PEG-LF还显示出长的循环时间和体内最高的肿瘤积累。最后,在小鼠异种移植模型上验证了980 nm光照射下GNRs的肿瘤消融能力,表明它们在NIR-II窗口中可能具有抗癌光热疗法。我们有机会通过肿瘤细胞,体外光热细胞毒性,体内血液循环和组织分布模式研究GNRs的尺寸和表面特性对其内在化的影响。结果,中等大小(长度为70 nm,宽度为11.5 nm)和表面PEG / LF修饰(GNR70 @ PEG-LF)的GNR在体外通过HepG2细胞表现出最快的细胞内在化和最佳光热结果。由于表面涂层和尺寸的协同作用,GNR70 @ PEG-LF还显示出长的循环时间和体内最高的肿瘤积累。最后,在小鼠异种移植模型上验证了980 nm光照射下GNRs的肿瘤消融能力,表明它们在NIR-II窗口中可能具有抗癌光热疗法。我们有机会通过肿瘤细胞,体外光热细胞毒性,体内血液循环和组织分布模式研究GNRs的尺寸和表面特性对其内在化的影响。结果,中等大小(长度为70 nm,宽度为11.5 nm)和表面PEG / LF修饰(GNR70 @ PEG-LF)的GNR在体外通过HepG2细胞表现出最快的细胞内在化和最佳光热结果。由于表面涂层和尺寸的协同作用,GNR70 @ PEG-LF还显示出长的循环时间和体内最高的肿瘤积累。最后,在小鼠异种移植模型上验证了980 nm光照射下GNRs的肿瘤消融能力,表明它们在NIR-II窗口中具有潜在的抗癌光热疗法。
更新日期:2020-01-13
中文翻译:

在第二个近红外窗口中,金纳米棒的大小和表面配体对肝癌积累和光热疗法的影响。
在第二近红外(NIR-II)窗口中具有纵向表面等离子体共振(LSPR)峰的金纳米棒(GNR)作为光热换能器吸引了众多关注,因为它们固有的优异的光热转换效率,高生物相容性和通用的表面功能化。这些GNRs在体内对肿瘤的应用的一个关键问题是哪种大小/形状和表面配体缀合有望用于循环和靶向肿瘤。在这项研究中,我们制备了一系列长宽比和LSPR峰相似的金纳米棒(GNR),因此在980 nm激光辐照下具有相似的光热传递效率,但宽度和长度均可调。将获得的GNR用PEG和肿瘤靶向配体乳铁蛋白进行表面修饰。有了这些量身定制的GNR,我们就有机会通过肿瘤细胞,体外光热细胞毒性,体内血液循环和组织分布模式研究GNR的尺寸和表面特性对其内在化的影响。结果,中等大小(长度为70 nm,宽度为11.5 nm)和表面PEG / LF修饰(GNR70 @ PEG-LF)的GNR在体外通过HepG2细胞表现出最快的细胞内在化和最佳光热结果。由于表面涂层和尺寸的协同作用,GNR70 @ PEG-LF还显示出长的循环时间和体内最高的肿瘤积累。最后,在小鼠异种移植模型上验证了980 nm光照射下GNRs的肿瘤消融能力,表明它们在NIR-II窗口中可能具有抗癌光热疗法。我们有机会通过肿瘤细胞,体外光热细胞毒性,体内血液循环和组织分布模式研究GNRs的尺寸和表面特性对其内在化的影响。结果,中等大小(长度为70 nm,宽度为11.5 nm)和表面PEG / LF修饰(GNR70 @ PEG-LF)的GNR在体外通过HepG2细胞表现出最快的细胞内在化和最佳光热结果。由于表面涂层和尺寸的协同作用,GNR70 @ PEG-LF还显示出长的循环时间和体内最高的肿瘤积累。最后,在小鼠异种移植模型上验证了980 nm光照射下GNRs的肿瘤消融能力,表明它们在NIR-II窗口中可能具有抗癌光热疗法。我们有机会通过肿瘤细胞,体外光热细胞毒性,体内血液循环和组织分布模式研究GNRs的尺寸和表面特性对其内在化的影响。结果,中等大小(长度为70 nm,宽度为11.5 nm)和表面PEG / LF修饰(GNR70 @ PEG-LF)的GNR在体外通过HepG2细胞表现出最快的细胞内在化和最佳光热结果。由于表面涂层和尺寸的协同作用,GNR70 @ PEG-LF还显示出长的循环时间和体内最高的肿瘤积累。最后,在小鼠异种移植模型上验证了980 nm光照射下GNRs的肿瘤消融能力,表明它们在NIR-II窗口中具有潜在的抗癌光热疗法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号