当前位置:
X-MOL 学术
›
J. Inorg. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tau/Aβ chimera peptides: Evaluating the dual function of metal coordination and membrane interaction in one sequence.
Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2020-01-11 , DOI: 10.1016/j.jinorgbio.2020.110996 Michele F M Sciacca 1 , Giuseppe Di Natale 1 , Rita Tosto 1 , Danilo Milardi 1 , Giuseppe Pappalardo 1
Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2020-01-11 , DOI: 10.1016/j.jinorgbio.2020.110996 Michele F M Sciacca 1 , Giuseppe Di Natale 1 , Rita Tosto 1 , Danilo Milardi 1 , Giuseppe Pappalardo 1
Affiliation
|
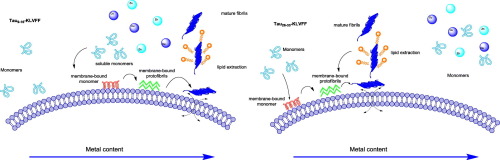
Several abnormal events may concur as major risk factors for Alzheimer's disease (AD) pathogenesis. For instance, dysregulation of brain's metal homeostasis and amyloid-mediated membrane damage are established toxic mechanisms causing neuronal death. In this study, we assess the amyloidogenic propensity and membrane-damage effects, either in the presence or in absence of metal ions, of two newly synthesized bifunctional peptides. These were designed to comprise a metal chelating N-terminus region derived from Tau protein namely the Tau9-16 (EVMEDHAG) or Tau26-33 (QGGYTMHQ) sequences, merged with the C-terminal hydrophobic region analogous to the Amyloid beta (Aβ) 16-20 aminoacid sequence KLVFF (KL). Comparative circular dichroism or fluorescence experiments were carried out to look at the peptide conformation, fibril formation and membrane affinity of Tau9-16KL and Tau26-33KL peptides. We found that Tau9-16KL and Tau26-33KL perturb the fibrillogenic process of Aβ1-40. Furthermore Cu(II) and, to a lower extent, Zn(II) induced conformational changes Tau26-33KL both in water and in membrane-mimicking environment. By contrast, due to a different metal coordination mode we observed for Tau9-16KL an unstructured peptide conformation in all the experimental conditions. Unlike aqueous solution, a certain propensity to form amyloid structures at the lipid membrane interface clearly emerged for both the peptides. However, the two peptides exhibit a different capability to elicit membrane damage depending on the presence or absence of metal ions. Tau9-16KL and Tau26-33KL can be used as peptide-based molecular systems able to interfere with the metal dependent Aβ/Tau cross-seeded generation of membrane active amyloid species.
中文翻译:

Tau /Aβ嵌合肽:以一个序列评估金属配位和膜相互作用的双重功能。
某些异常事件可能是阿尔茨海默氏病(AD)发病机理的主要危险因素。例如,大脑金属稳态的失调和淀粉样蛋白介导的膜损伤是导致神经元死亡的毒性机制。在这项研究中,我们评估了两种新合成的双功能肽在存在或不存在金属离子的情况下的淀粉样变性倾向和膜破坏作用。这些被设计为包含源自Tau蛋白的金属螯合N末端区域,即Tau9-16(EVMEDHAG)或Tau26-33(QGGYTMHQ)序列,并与类似于淀粉样β(Aβ)16的C端疏水区合并-20个氨基酸序列KLVFF(KL)。进行了比较圆二色性或荧光实验以观察肽构象,Tau9-16KL和Tau26-33KL肽的原纤维形成和膜亲和力。我们发现Tau9-16KL和Tau26-33KL扰乱了Aβ1-40的原纤维形成过程。此外,在水中和模拟膜的环境中,Cu(II)和较低程度的Zn(II)诱导构象变化Tau26-33KL。相比之下,由于不同的金属配位模式,我们在所有实验条件下都观察到了Tau9-16KL的非结构化肽构象。与水溶液不同,两种肽在脂质膜界面形成淀粉样蛋白结构都有一定的倾向。然而,取决于金属离子的存在或不存在,两种肽表现出引起膜损伤的不同能力。
更新日期:2020-01-13
中文翻译:

Tau /Aβ嵌合肽:以一个序列评估金属配位和膜相互作用的双重功能。
某些异常事件可能是阿尔茨海默氏病(AD)发病机理的主要危险因素。例如,大脑金属稳态的失调和淀粉样蛋白介导的膜损伤是导致神经元死亡的毒性机制。在这项研究中,我们评估了两种新合成的双功能肽在存在或不存在金属离子的情况下的淀粉样变性倾向和膜破坏作用。这些被设计为包含源自Tau蛋白的金属螯合N末端区域,即Tau9-16(EVMEDHAG)或Tau26-33(QGGYTMHQ)序列,并与类似于淀粉样β(Aβ)16的C端疏水区合并-20个氨基酸序列KLVFF(KL)。进行了比较圆二色性或荧光实验以观察肽构象,Tau9-16KL和Tau26-33KL肽的原纤维形成和膜亲和力。我们发现Tau9-16KL和Tau26-33KL扰乱了Aβ1-40的原纤维形成过程。此外,在水中和模拟膜的环境中,Cu(II)和较低程度的Zn(II)诱导构象变化Tau26-33KL。相比之下,由于不同的金属配位模式,我们在所有实验条件下都观察到了Tau9-16KL的非结构化肽构象。与水溶液不同,两种肽在脂质膜界面形成淀粉样蛋白结构都有一定的倾向。然而,取决于金属离子的存在或不存在,两种肽表现出引起膜损伤的不同能力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号