Dyes and Pigments ( IF 4.5 ) Pub Date : 2020-01-11 , DOI: 10.1016/j.dyepig.2020.108194 Hoa Thi Bui , Jong Min Lim , Duy Khuong Mai , Heemon Kim , Ho-Joong Kim , Hyung Jin Kim , Sung Cho
|
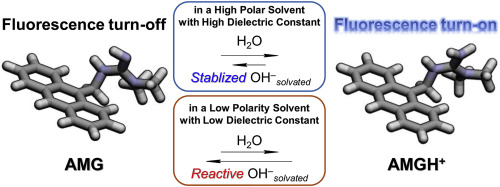
We investigated the mechanism and sensing efficiency of an anthracene-based fluorescent water-content chemosensor (AMG) containing a guanidine moiety in water-miscible organic solvents. Based on spectroscopic and theoretical results, we established that there are two functions of water for AMG in organic solvents, one of which is as a reagent for protonation and the other is in the solvation and stabilization of ionic products, such as protonated AMG (AMGH+) and hydroxide ion. Moreover, the effective solvation and stabilization of ionic products in organic solvents are critical for the mitigation of the deprotonation between them and the relatively high ratio of the fluorescence turn-on AMGH+ species. In 1,4-dioxane with low polarity, AMGH+ and hydroxide ion cannot be effectively solvated, and there is a strong positive correlation between the fluorescence intensity and volumetric fraction of water due to the improved solvation condition caused by the co-existent water. Conversely, the amount of water in polar solvents is less significant, as evidenced by the relatively small increase in the fluorescence intensity upon increasing the water content. This is because ionic products can be stabilized by polar organic solvent molecules without cooperation with water. Conclusively, the solvation and stabilization of ionic products produced by a chemical reaction at a chemical sensing site in a fluorescent chemosensor are important for determining the relative ratio between the fluorescence turn-on and –off species and the overall sensing efficiency of the fluorescent chemosensors.
中文翻译:

荧光含水化学传感器在有机溶剂中的溶剂化和稳定化
我们研究了在可与水混溶的有机溶剂中包含胍基的蒽基荧光水化学传感器(AMG)的机理和传感效率。根据光谱和理论结果,我们确定了AMG在有机溶剂中的水具有两种功能,一种是质子化试剂,另一种是离子产品(如质子化AMG(AMGH)的溶剂化和稳定化)。+)和氢氧根离子。此外,离子产物在有机溶剂中的有效溶剂化和稳定化对于缓解它们之间的去质子化和相对较高的荧光开启AMGH +比例至关重要。种类。在低极性1,4-二恶烷中,AMGH +氢氧根离子不能有效地被溶剂化,并且由于水的共存导致溶剂化条件的改善,因此荧光强度与水的体积分数之间存在很强的正相关关系。相反,极性溶剂中的水量不那么重要,这可以通过增加水含量后荧光强度的相对较小的增加来证明。这是因为离子产品可以通过极性有机溶剂分子稳定,而无需与水合作。总之,在荧光化学传感器中,由化学反应在化学传感位点发生的化学反应产生的离子产物的溶剂化和稳定化对于确定荧光开启和关闭物种之间的相对比例以及荧光化学传感器的整体传感效率至关重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号