当前位置:
X-MOL 学术
›
Spectrochim. Acta. A Mol. Biomol. Spectrosc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Evaluation of ct-DNA and HSA binding propensity of antibacterial drug chloroxine: Multi-spectroscopic analysis, atomic force microscopy and docking simulation.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.4 ) Pub Date : 2020-01-10 , DOI: 10.1016/j.saa.2020.118042 Nahid Shahabadi 1 , Saba Zendehcheshm 2
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.4 ) Pub Date : 2020-01-10 , DOI: 10.1016/j.saa.2020.118042 Nahid Shahabadi 1 , Saba Zendehcheshm 2
Affiliation
|
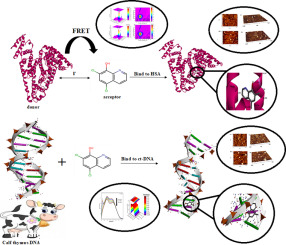
In the present study, the binding interactions of chloroxine, an antibacterial drug and antibiotic agent with calf thymus-deoxyribonucleic acid (ct-DNA) and human serum albumin (HSA) have been deliberated under simulative physiological conditions (pH = 7.40) employing multiple biophysical, atomic force microscopy and molecular modeling approaches. The ct-DNA binding properties of chloroxine exhibit that it binds to ct-DNA through a groove binding mode, and the binding constant values were computed employing the absorption and emission spectral data. The fluorescence study shows the presence of the static quenching mechanism in the ct-DNA- chloroxine interaction. These results are further supported by UV-vis spectra. Large complexes contain the ct-DNA chains with an average size of 225.45 nm were observed by employing AFM for chloroxine -ct-DNA. The results revealed that the fluorescence quenching of albumin by chloroxine was a static quenching process as a result of albumin-chloroxine (1:1) complex. The distance between chloroxine and albumin was obtained based on the Förster's theory of non-radiative energy transfer. The results of AFM, synchronous and three-dimensional fluorescence spectra all revealed that chloroxine induced the conformational changes of albumin. Molecular docking technology represents the binding of chloroxine to the major groove of ct-DNA and site I (subdomain II A) of albumin.
中文翻译:

抗菌药物氯辛的ct-DNA和HSA结合倾向的评估:多光谱分析,原子力显微镜和对接模拟。
在本研究中,在模拟生理条件下(pH = 7.40),采用多种生物物理方法,研究了抗菌素和抗生素氯辛与小牛胸腺-脱氧核糖核酸(ct-DNA)和人血清白蛋白(HSA)的结合相互作用。 ,原子力显微镜和分子建模方法。氯辛的ct-DNA结合特性表明它通过凹槽结合模式与ct-DNA结合,结合常数值使用吸收和发射光谱数据计算得出。荧光研究表明,在ct-DNA-氯嗪相互作用中存在静态猝灭机制。紫外可见光谱进一步支持了这些结果。通过将AFM用作氯辛-ct-DNA,观察到大复合物包含ct-DNA链,平均链长为225.45 nm。结果表明,氯霉素对白蛋白的荧光猝灭是静态的猝灭过程,这是白蛋白-氯辛(1:1)络合物的结果。根据Förster的非辐射能量转移理论获得了氯辛与白蛋白之间的距离。AFM,同步和三维荧光光谱的结果均表明,氯辛诱导了白蛋白的构象变化。分子对接技术代表氯氧嘧啶与ct-DNA的主要凹槽和白蛋白的I位(亚域II A)结合。AFM,同步和三维荧光光谱的结果均表明,氯辛诱导了白蛋白的构象变化。分子对接技术代表氯氧嘧啶与ct-DNA的主要凹槽和白蛋白的I位(亚域II A)结合。AFM,同步和三维荧光光谱的结果均表明,氯辛诱导了白蛋白的构象变化。分子对接技术代表氯氧嘧啶与ct-DNA的主要凹槽和白蛋白的I位(亚域II A)结合。
更新日期:2020-01-11
中文翻译:

抗菌药物氯辛的ct-DNA和HSA结合倾向的评估:多光谱分析,原子力显微镜和对接模拟。
在本研究中,在模拟生理条件下(pH = 7.40),采用多种生物物理方法,研究了抗菌素和抗生素氯辛与小牛胸腺-脱氧核糖核酸(ct-DNA)和人血清白蛋白(HSA)的结合相互作用。 ,原子力显微镜和分子建模方法。氯辛的ct-DNA结合特性表明它通过凹槽结合模式与ct-DNA结合,结合常数值使用吸收和发射光谱数据计算得出。荧光研究表明,在ct-DNA-氯嗪相互作用中存在静态猝灭机制。紫外可见光谱进一步支持了这些结果。通过将AFM用作氯辛-ct-DNA,观察到大复合物包含ct-DNA链,平均链长为225.45 nm。结果表明,氯霉素对白蛋白的荧光猝灭是静态的猝灭过程,这是白蛋白-氯辛(1:1)络合物的结果。根据Förster的非辐射能量转移理论获得了氯辛与白蛋白之间的距离。AFM,同步和三维荧光光谱的结果均表明,氯辛诱导了白蛋白的构象变化。分子对接技术代表氯氧嘧啶与ct-DNA的主要凹槽和白蛋白的I位(亚域II A)结合。AFM,同步和三维荧光光谱的结果均表明,氯辛诱导了白蛋白的构象变化。分子对接技术代表氯氧嘧啶与ct-DNA的主要凹槽和白蛋白的I位(亚域II A)结合。AFM,同步和三维荧光光谱的结果均表明,氯辛诱导了白蛋白的构象变化。分子对接技术代表氯氧嘧啶与ct-DNA的主要凹槽和白蛋白的I位(亚域II A)结合。



























 京公网安备 11010802027423号
京公网安备 11010802027423号