当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Low-temperature catalytic oxidation of benzene over nanocrystalline Cu–Mn composite oxides by facile sol–gel synthesis
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2020/01/10 , DOI: 10.1039/c9nj05097c Min Zhang 1, 2, 3, 4, 5 , Weiman Li 1, 2, 3, 4, 5 , Xiaofeng Wu 1, 2, 3, 4, 5 , Feng Zhao 1, 2, 3, 4, 5 , Dongdong Wang 1, 2, 3, 4, 5 , Xicuo Zha 1, 2, 3, 4, 5 , Shuangde Li 1, 2, 3, 4, 5 , Haidi Liu 1, 2, 3, 4, 5 , Yunfa Chen 1, 2, 3, 4, 5
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2020/01/10 , DOI: 10.1039/c9nj05097c Min Zhang 1, 2, 3, 4, 5 , Weiman Li 1, 2, 3, 4, 5 , Xiaofeng Wu 1, 2, 3, 4, 5 , Feng Zhao 1, 2, 3, 4, 5 , Dongdong Wang 1, 2, 3, 4, 5 , Xicuo Zha 1, 2, 3, 4, 5 , Shuangde Li 1, 2, 3, 4, 5 , Haidi Liu 1, 2, 3, 4, 5 , Yunfa Chen 1, 2, 3, 4, 5
Affiliation
|
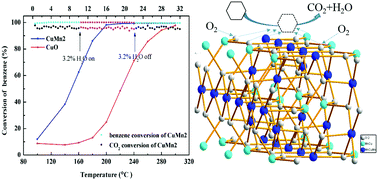
A series of nanocrystalline copper–manganese oxides (denoted as Cu3−xMnx, x = 0, 1, 1.5, 2, 2.5, 3, where x means the molar ratio of Cu and Mn) were successfully prepared by a facile citric acid sol–gel method. The combination of Cu2+ and Mn3+ is intensified and enhanced interface effects generated, which is beneficial for the catalytic oxidation of benzene. A series of analyses, such as X-Ray Diffraction (XRD), N2 adsorption–desorption, X-ray photoelectron spectroscopy (XPS), and hydrogen temperature programmed reduction (H2-TPR), were employed to further investigate the structural properties of the catalysts. An optimal Mn/Cu ratio of 2 forms CuMn2O4 spinels. CuMn2 with CuMn2O4 spinel structure presents a larger specific surface area, smaller pore diameter as well as more lattice oxygen species, exhibiting remarkable activity and stability for the catalytic oxidation of benzene. On account of these factors, CuMn2 possesses better low temperature reducibility and shows the best catalytic performance with 90% benzene conversion at 186 °C. The enhanced catalytic activity of CuMn2 is attributed to the stabilization of CuMn2O4 active phases and the intensive synergistic effect between Cu–Mn oxides. To prove the effect of CuMn2O4 spinel structure on catalytic performance, a CuO/Mn2O3 mixed catalyst (molar ratio 1 : 1) was prepared and applied to benzene oxidation (T90% = 198 °C), which indicates that the spinel structure has an encouraging effect on benzene catalysis. The catalytic properties of single copper oxide and manganese trioxide were also tested, the results show that CuMn2O4 has a crucial role in facilitating electronic transmission and mobility of the lattice oxygen.
中文翻译:

溶胶-凝胶法合成纳米晶Cu-Mn复合氧化物上苯的低温催化氧化
通过简便的柠檬酸法成功制备了一系列纳米晶态的铜-锰氧化物(表示为Cu 3- x Mn x,x = 0、1、1.5、2、2.5、3,其中x表示Cu和Mn的摩尔比)酸溶胶凝胶法。Cu 2+和Mn 3+的结合被增强并产生增强的界面效应,这对于苯的催化氧化是有利的。一系列分析,例如X射线衍射(XRD),N 2吸附-解吸,X射线光电子能谱(XPS)和氢程序升温还原(H 2-TPR)被用于进一步研究催化剂的结构性质。Mn / Cu的最佳比例为2会形成CuMn 2 O 4尖晶石。的CuMn 2与的CuMn 2 ö 4尖晶石结构呈现出较大的比表面积,更小的孔径以及多个晶格氧,表现出显着的活性和稳定性为苯的催化氧化。由于这些因素,CuMn 2具有更好的低温还原性,并在186°C时具有90%的苯转化率表现出最佳的催化性能。CuMn 2的催化活性增强归因于CuMn 2 O 4的稳定活性相和Cu-Mn氧化物之间强烈的协同作用。为了证明CuMn 2 O 4尖晶石结构对催化性能的影响,制备了CuO / Mn 2 O 3混合催化剂(摩尔比为1:1),并用于苯氧化(T 90% = 198°C),表明尖晶石结构对苯催化具有令人鼓舞的作用。还测试了单一氧化铜和三氧化锰的催化性能,结果表明CuMn 2 O 4在促进电子传输和晶格氧的迁移方面具有关键作用。
更新日期:2020-02-12
中文翻译:

溶胶-凝胶法合成纳米晶Cu-Mn复合氧化物上苯的低温催化氧化
通过简便的柠檬酸法成功制备了一系列纳米晶态的铜-锰氧化物(表示为Cu 3- x Mn x,x = 0、1、1.5、2、2.5、3,其中x表示Cu和Mn的摩尔比)酸溶胶凝胶法。Cu 2+和Mn 3+的结合被增强并产生增强的界面效应,这对于苯的催化氧化是有利的。一系列分析,例如X射线衍射(XRD),N 2吸附-解吸,X射线光电子能谱(XPS)和氢程序升温还原(H 2-TPR)被用于进一步研究催化剂的结构性质。Mn / Cu的最佳比例为2会形成CuMn 2 O 4尖晶石。的CuMn 2与的CuMn 2 ö 4尖晶石结构呈现出较大的比表面积,更小的孔径以及多个晶格氧,表现出显着的活性和稳定性为苯的催化氧化。由于这些因素,CuMn 2具有更好的低温还原性,并在186°C时具有90%的苯转化率表现出最佳的催化性能。CuMn 2的催化活性增强归因于CuMn 2 O 4的稳定活性相和Cu-Mn氧化物之间强烈的协同作用。为了证明CuMn 2 O 4尖晶石结构对催化性能的影响,制备了CuO / Mn 2 O 3混合催化剂(摩尔比为1:1),并用于苯氧化(T 90% = 198°C),表明尖晶石结构对苯催化具有令人鼓舞的作用。还测试了单一氧化铜和三氧化锰的催化性能,结果表明CuMn 2 O 4在促进电子传输和晶格氧的迁移方面具有关键作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号