当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Properties of Aqueous Trehalose Mixtures: Glass Transition and Hydrogen Bonding.
Journal of Chemical Theory and Computation ( IF 5.5 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.jctc.9b01071 Gil I Olgenblum 1 , Liel Sapir 2 , Daniel Harries 1
Journal of Chemical Theory and Computation ( IF 5.5 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.jctc.9b01071 Gil I Olgenblum 1 , Liel Sapir 2 , Daniel Harries 1
Affiliation
|
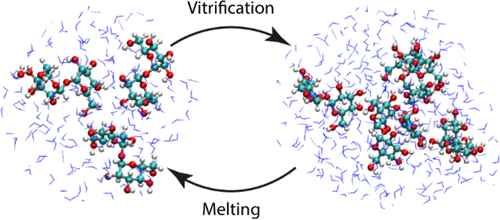
Trehalose is a naturally occurring disaccharide known to remarkably stabilize biomacromolecules in the biologically active state. The stabilizing effect is typically observed over a large concentration range and affects many macromolecules including proteins, lipids, and DNA. Of special interest is the transition from aqueous solution to the dense and highly concentrated glassy state of trehalose that has been implicated in bioadaptation of different organisms toward desiccation stress. Although several mechanisms have been suggested to link the structure of the low water content glass with its action as an exceptional stabilizer, studies are ongoing to resolve which are most pertinent. Specifically, the role that hydrogen bonding plays in the formation of the glass is not well resolved. Here we model aqueous trehalose mixtures over a wide concentration range, using molecular dynamics simulations with two available force fields. Both force fields indicate glass transition temperatures and osmotic pressures that are close to experimental values, particularly at high trehalose contents. We develop and employ a methodology that allows us to analyze the thermodynamics of hydrogen bonds in simulations at different water contents and temperatures. Remarkably, this analysis is able to link the liquid to glass transition with changes in hydrogen bond characteristics. Most notably, the onset of the glassy state can be quantitatively related to the transition from weakly to strongly correlated hydrogen bonds. Our findings should help resolve the properties of the glass and the mechanisms of its formation in the presence of added macromolecules.
中文翻译:

海藻糖水性混合物的性质:玻璃化转变和氢键。
海藻糖是一种天然存在的二糖,已知可以将生物大分子稳定在生物活性状态。通常在较大的浓度范围内观察到稳定作用,并影响许多大分子,包括蛋白质,脂质和DNA。特别令人感兴趣的是从水溶液到海藻糖的致密且高度浓缩的玻璃态的转变,这与不同生物对干燥胁迫的生物适应有关。尽管已经提出了几种机制将低水玻璃的结构与其作为特殊稳定剂的作用联系起来,但仍在进行研究以解决最相关的问题。具体而言,不能很好地解决氢键在玻璃形成中的作用。在此,我们使用具有两个可用力场的分子动力学模拟对宽浓度范围内的海藻糖水溶液混合物进行建模。这两个力场都表明玻璃化转变温度和渗透压接近实验值,尤其是在海藻糖含量高的情况下。我们开发并采用了一种方法,使我们能够在不同的水含量和温度下模拟分析氢键的热力学。值得注意的是,这种分析能够将液体与玻璃的转变与氢键特征的变化联系起来。最值得注意的是,玻璃态的发生可以与从弱相关到强相关氢键的转变定量相关。
更新日期:2020-01-24
中文翻译:

海藻糖水性混合物的性质:玻璃化转变和氢键。
海藻糖是一种天然存在的二糖,已知可以将生物大分子稳定在生物活性状态。通常在较大的浓度范围内观察到稳定作用,并影响许多大分子,包括蛋白质,脂质和DNA。特别令人感兴趣的是从水溶液到海藻糖的致密且高度浓缩的玻璃态的转变,这与不同生物对干燥胁迫的生物适应有关。尽管已经提出了几种机制将低水玻璃的结构与其作为特殊稳定剂的作用联系起来,但仍在进行研究以解决最相关的问题。具体而言,不能很好地解决氢键在玻璃形成中的作用。在此,我们使用具有两个可用力场的分子动力学模拟对宽浓度范围内的海藻糖水溶液混合物进行建模。这两个力场都表明玻璃化转变温度和渗透压接近实验值,尤其是在海藻糖含量高的情况下。我们开发并采用了一种方法,使我们能够在不同的水含量和温度下模拟分析氢键的热力学。值得注意的是,这种分析能够将液体与玻璃的转变与氢键特征的变化联系起来。最值得注意的是,玻璃态的发生可以与从弱相关到强相关氢键的转变定量相关。


























 京公网安备 11010802027423号
京公网安备 11010802027423号