Carbon ( IF 10.9 ) Pub Date : 2020-01-10 , DOI: 10.1016/j.carbon.2020.01.011 Andrea M. Oyarzún , Ximena García-Carmona , Ljubisa R. Radovic
|
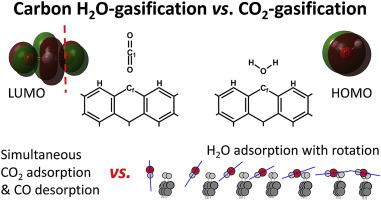
This computational chemistry analysis compares the interactions of H2O and CO2 with zigzag graphene edges, and in particular their adsorption and desorption steps. We provide detailed information regarding the geometric and electronic factors that influence both their adsorption and desorption (of H2 and CO) processes. Density functional theory results are compared with experimental information to offer heretofore unavailable insights into key aspects of the rate-limiting steps, inhibition phenomena and nanoscale differences in these two reactions. Thus, for example, the orientation and rotation of the adsorbing H2O molecules are elucidated using intrinsic reaction coordinate calculations and molecular orbital analysis, and these results complement recent pulse field gradient NMR measurements and molecular dynamics calculations. Such mechanistic findings reveal the H2O adsorption process to be a slow rotational phenomenon whereas the low-temperature H2 desorption is geometrically constrained by the presence of contiguous (di)hydrogenated carbon atoms with or without hydroxyl groups. This surface-assisted desorption mechanism is proposed to be responsible for the formation of hexagonal pits during the graphite-H2O reaction. Similar insights were obtained for the graphene-CO2 reaction. Mechanistic schemes are proposed for the desorption products observed in experiments, distinguishing zigzag from armchair sites.
中文翻译:

石墨烯表面上氧转移反应的动力学。第二部分 H 2 o与Co 2
该计算化学分析比较了H 2 O和CO 2与之字形石墨烯边缘的相互作用,特别是它们的吸附和解吸步骤。我们提供有关影响其H 2和CO吸附和解吸的几何和电子因素的详细信息。将密度泛函理论的结果与实验信息进行比较,以提供迄今为止对这两个反应中限速步骤,抑制现象和纳米级差异的关键方面尚无可用的见解。因此,例如,吸附H 2的取向和旋转使用本征反应坐标计算和分子轨道分析来阐明O分子,这些结果补充了最近的脉冲场梯度NMR测量和分子动力学计算。这种机理上的发现表明,H 2 O的吸附过程是一个缓慢的旋转现象,而低温H 2的解吸在几何上受到具有或不具有羟基的连续的(二)氢化碳原子的限制。有人提出这种表面辅助解吸机制负责在石墨-H 2 O反应过程中形成六边形凹坑。对于石墨烯-CO 2也获得了类似的见解。反应。针对在实验中观察到的解吸产物,提出了机械方案,以区分锯齿形和扶手椅形。


























 京公网安备 11010802027423号
京公网安备 11010802027423号