Dyes and Pigments ( IF 4.5 ) Pub Date : 2020-01-10 , DOI: 10.1016/j.dyepig.2020.108193 Felipe Lange Coelho , Rodrigo da Costa Duarte , Cláudia de Ávila Braga , Josene Maria Toldo , Paulo Fernando Bruno Gonçalves , Fabiano da Silveira Santos , Fabiano Severo Rodembusch
|
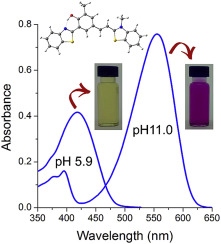
Substituted merocyanine dyes were obtained by Knoevenagel condensation between formylated arylbenzothiazole and quaternary 2-alkylbenzothiazoles. The condensations were performed in the presence of sodium methoxide and the products were obtained with excellent yields after simple purification. The merocyanines presented absorption maxima in the range 576–605 nm (green to orange-red region). The calculated Strickler-Berg parameters indicated fully spin and symmetry allowed electronic transitions with 1π-π* character. These compounds show significant fluorescence emission at the orange red region with a very small Stokes shift. Surprisingly, although the benzothiazole core possesses a structural moiety (o-hydroxyaryl moiety) which should facilitate proton transfer in the excited state, no evidence was found of this phototautomerism in the compounds studied. TD-DFT calculations show that ESIPT is not probable to happen due to the high barriers to transfer the proton. In addition, the aliphatic chain does not affect the absorption and emission maxima location. For both, ground and excited states, a negative solvatochromic effect was observed. Theoretical calculations also show that this effect is due to the larger dipole moment in the S0 than in the S1 state. In addition, these compounds presented large shifts on the absorption spectra changing the pH (yellow to violet color), which allowed studying successfully as optical sensor for middle pH values.
中文翻译:

苯并噻唑花青染料用作中等pH值光学传感器
通过甲酰化的芳基苯并噻唑和季2-烷基苯并噻唑之间的Knoevenagel缩合获得取代的花菁染料。在甲醇钠的存在下进行缩合,并且在简单纯化后以优异的产率获得产物。花青素的最大吸收范围为576-605 nm(绿色至橙红色区域)。所计算出的斯特里克勒伯格参数所表示完全旋转对称并允许与电子跃迁1 π-π*字符。这些化合物在橙红色区域显示出显着的荧光发射,并且斯托克斯位移很小。令人惊讶地,虽然苯并噻唑芯具有的结构部分(Ô-羟基芳基部分)(应促进质子在激发态下的转移),在所研究的化合物中未发现这种光互变异构现象的证据。TD-DFT计算表明,由于转移质子的障碍较高,因此不太可能发生ESIPT。另外,脂族链不影响最大吸收和发射位置。对于基态和激发态,均观察到负溶剂变色效应。理论计算还表明,这种影响是由于S 0中的偶极矩比S 1状态中的偶极矩大。此外,这些化合物在吸收光谱上呈现出很大的变化,从而改变了pH值(从黄色到紫色),这使得它可以作为光学传感器成功地研究中pH值。

























 京公网安备 11010802027423号
京公网安备 11010802027423号