Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Conformational flexibility of GRASPs and their constituent PDZ subdomains reveals structural basis of their promiscuous interactome.
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-01-09 , DOI: 10.1111/febs.15206 Luis Felipe S Mendes 1, 2 , Mariana R B Batista 1 , Peter J Judge 2 , Anthony Watts 2 , Christina Redfield 2 , Antonio J Costa-Filho 1
The FEBS Journal ( IF 5.4 ) Pub Date : 2020-01-09 , DOI: 10.1111/febs.15206 Luis Felipe S Mendes 1, 2 , Mariana R B Batista 1 , Peter J Judge 2 , Anthony Watts 2 , Christina Redfield 2 , Antonio J Costa-Filho 1
Affiliation
|
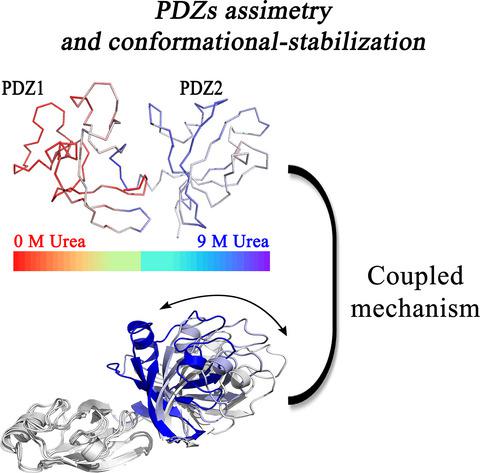
The Golgi complex is a central component of the secretory pathway, responsible for several critical cellular functions in eukaryotes. The complex is organized by the Golgi matrix that includes the Golgi reassembly and stacking protein (GRASP), which was shown to be involved in cisternae stacking and lateral linkage in metazoan. GRASPs also have critical roles in other processes, with an unusual ability to interact with several different binding partners. The conserved N terminus of the GRASP family includes two PSD‐95, DLG, and ZO‐1 (PDZ) domains. Previous crystallographic studies of orthologues suggest that PDZ1 and PDZ2 have similar conformations and secondary structure content. However, PDZ1 alone mediates nearly all interactions between GRASPs and their partners. In this work, NMR, synchrotron radiation CD, and molecular dynamics (MD) were used to examine the structure, flexibility, and stability of the two constituent PDZ domains. GRASP PDZs are structured in an unusual β3α1β4β5α2β6β1β2 secondary structural arrangement and NMR data indicate that the PDZ1 binding pocket is formed by a stable β2‐strand and a more flexible and unstable α2‐helix, suggesting an explanation for the higher PDZ1 promiscuity. The conformational free energy profiles of the two PDZ domains were calculated using MD simulations. The data suggest that, after binding, the protein partner significantly reduces the conformational space that GRASPs can access by stabilizing one particular conformation, in a partner‐dependent fashion. The structural flexibility of PDZ1, modulated by PDZ2, and the coupled, coordinated movement between the two PDZs enable GRASPs to interact with multiple partners, allowing them to function as promiscuous, multitasking proteins.
中文翻译:

GRASPs及其组成的PDZ子域的构象灵活性揭示了其混杂相互作用组的结构基础。
高尔基复合体是分泌途径的重要组成部分,负责真核生物的几种关键细胞功能。该复合物由包括高尔基体重组和堆积蛋白(GRASP)的高尔基矩阵组成,该蛋白被证明与后生动物的水箱堆叠和侧链有关。GRASP在其他过程中也起着关键作用,具有与几个不同的绑定伙伴进行交互的非同寻常的能力。GRASP系列的保守N末端包括两个PSD-95,DLG和ZO-1(PDZ)域。以前的直向同源物的晶体学研究表明,PDZ1和PDZ2具有相似的构象和二级结构含量。但是,仅PDZ1会介导GRASP及其伙伴之间的几乎所有交互。在这项工作中,NMR,同步辐射CD 和分子动力学(MD)用于检查两个组成的PDZ域的结构,柔性和稳定性。GRASP PDZ的结构具有异常的β3 α 1 β 4 β 5 α 2 β 6 β 1 β 2个的二级结构排列和NMR数据表明,该PDZ1结合口袋由稳定的β形成2 -链和更灵活的和不稳定的α 2-螺旋线,提示PDZ1混杂度较高。使用MD模拟计算两个PDZ域的构象自由能分布。数据表明,结合后,蛋白伴侣以依赖伴侣的方式稳定一种特定构象,从而大大降低了GRASPs可以进入的构象空间。由PDZ2调节的PDZ1的结构灵活性以及两个PDZ之间的耦合,协调的运动使GRASP与多个伙伴相互作用,从而使其可以充当混杂的多任务蛋白。
更新日期:2020-01-09
中文翻译:

GRASPs及其组成的PDZ子域的构象灵活性揭示了其混杂相互作用组的结构基础。
高尔基复合体是分泌途径的重要组成部分,负责真核生物的几种关键细胞功能。该复合物由包括高尔基体重组和堆积蛋白(GRASP)的高尔基矩阵组成,该蛋白被证明与后生动物的水箱堆叠和侧链有关。GRASP在其他过程中也起着关键作用,具有与几个不同的绑定伙伴进行交互的非同寻常的能力。GRASP系列的保守N末端包括两个PSD-95,DLG和ZO-1(PDZ)域。以前的直向同源物的晶体学研究表明,PDZ1和PDZ2具有相似的构象和二级结构含量。但是,仅PDZ1会介导GRASP及其伙伴之间的几乎所有交互。在这项工作中,NMR,同步辐射CD 和分子动力学(MD)用于检查两个组成的PDZ域的结构,柔性和稳定性。GRASP PDZ的结构具有异常的β3 α 1 β 4 β 5 α 2 β 6 β 1 β 2个的二级结构排列和NMR数据表明,该PDZ1结合口袋由稳定的β形成2 -链和更灵活的和不稳定的α 2-螺旋线,提示PDZ1混杂度较高。使用MD模拟计算两个PDZ域的构象自由能分布。数据表明,结合后,蛋白伴侣以依赖伴侣的方式稳定一种特定构象,从而大大降低了GRASPs可以进入的构象空间。由PDZ2调节的PDZ1的结构灵活性以及两个PDZ之间的耦合,协调的运动使GRASP与多个伙伴相互作用,从而使其可以充当混杂的多任务蛋白。

























 京公网安备 11010802027423号
京公网安备 11010802027423号