当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Analysis of Proline Substitutions Reveals the Plasticity and Sequence Sensitivity of Human IAPP Amyloidogenicity and Toxicity.
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.biochem.9b01109 Zachary Ridgway 1 , Charles Eldrid 2 , Alexander Zhyvoloup 2 , Aisha Ben-Younis 2 , Daeun Noh 3 , Konstantinos Thalassinos 2 , Daniel P Raleigh 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.biochem.9b01109 Zachary Ridgway 1 , Charles Eldrid 2 , Alexander Zhyvoloup 2 , Aisha Ben-Younis 2 , Daeun Noh 3 , Konstantinos Thalassinos 2 , Daniel P Raleigh 1, 2
Affiliation
|
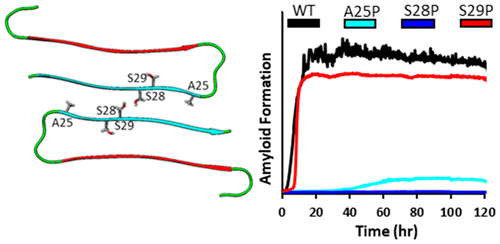
Pancreatic amyloid formation by the polypeptide IAPP contributes to β-cell dysfunction in type 2 diabetes. There is a 1:1 correspondence between the ability of IAPP from different species to form amyloid in vitro and the susceptibility of the organism to develop diabetes. Rat IAPP is non-amyloidogenic and differs from human IAPP at six positions, including three proline replacements: A25P, S28P, and S29P. Incorporation of these proline residues into human IAPP leads to a non-amyloidogenic analogue that is used clinically. The role of the individual proline residues is not understood. We examine the three single and three double proline substitutions in the context of human IAPP. An S28P substitution significantly decreases amyloidogenicity and toxicity, while an S29P substitution has very modest effects despite being an identical replacement just one residue away. The consequences of the A25P substitution are between those of the two Ser to Pro substitutions. Double analogues containing an S28P replacement are less amyloidogenic and less toxic than the IAPPA25P S29P double analogue. Ion mobility mass spectrometry reveals that there is no correlation between the monomer or dimer conformation as reported by collision cross section measurements and the time to form amyloid. The work reveals both the plasticity of IAPP amyloid formation and the exquisite sequence sensitivity of IAPP amyloidogenicity and toxicity. The study highlights the key role of the S28P substitution and provides information that will aid in the rational design of soluble variants of IAPP. The variants studied here offer a system for further exploring features that control IAPP toxicity.
中文翻译:

脯氨酸取代的分析揭示了人类IAPP淀粉样变性和毒性的可塑性和序列敏感性。
多肽IAPP形成的胰腺淀粉样蛋白可导致2型糖尿病的β细胞功能异常。不同物种的IAPP在体外形成淀粉样蛋白的能力与生物体患糖尿病的易感性之间存在1:1的对应关系。大鼠IAPP具有非淀粉样生成作用,在六个位置与人IAPP不同,包括三个脯氨酸替代物:A25P,S28P和S29P。将这些脯氨酸残基掺入人IAPP会导致临床上不会产生淀粉样蛋白。各个脯氨酸残基的作用尚不清楚。我们在人类IAPP的背景下研究了三个单脯氨酸和三个双脯氨酸替代。S28P取代可显着降低淀粉样变性和毒性,尽管S29P替代品是相同的替代品,但仅相距一个残基,其作用却非常适中。A25P取代的后果介于两个Ser到Pro取代之间。与IAPPA25P S29P双重类似物相比,含有S28P替代物的双重类似物具有更少的淀粉样蛋白生成和更低的毒性。离子迁移质谱表明,通过碰撞截面测量报告的单体或二聚体构象与形成淀粉样蛋白的时间之间没有相关性。这项工作揭示了IAPP淀粉样蛋白形成的可塑性以及IAPP淀粉样蛋白形成性和毒性的出色序列敏感性。该研究强调了S28P替代的关键作用,并提供了有助于合理设计IAPP可溶性变体的信息。
更新日期:2020-01-31
中文翻译:

脯氨酸取代的分析揭示了人类IAPP淀粉样变性和毒性的可塑性和序列敏感性。
多肽IAPP形成的胰腺淀粉样蛋白可导致2型糖尿病的β细胞功能异常。不同物种的IAPP在体外形成淀粉样蛋白的能力与生物体患糖尿病的易感性之间存在1:1的对应关系。大鼠IAPP具有非淀粉样生成作用,在六个位置与人IAPP不同,包括三个脯氨酸替代物:A25P,S28P和S29P。将这些脯氨酸残基掺入人IAPP会导致临床上不会产生淀粉样蛋白。各个脯氨酸残基的作用尚不清楚。我们在人类IAPP的背景下研究了三个单脯氨酸和三个双脯氨酸替代。S28P取代可显着降低淀粉样变性和毒性,尽管S29P替代品是相同的替代品,但仅相距一个残基,其作用却非常适中。A25P取代的后果介于两个Ser到Pro取代之间。与IAPPA25P S29P双重类似物相比,含有S28P替代物的双重类似物具有更少的淀粉样蛋白生成和更低的毒性。离子迁移质谱表明,通过碰撞截面测量报告的单体或二聚体构象与形成淀粉样蛋白的时间之间没有相关性。这项工作揭示了IAPP淀粉样蛋白形成的可塑性以及IAPP淀粉样蛋白形成性和毒性的出色序列敏感性。该研究强调了S28P替代的关键作用,并提供了有助于合理设计IAPP可溶性变体的信息。



























 京公网安备 11010802027423号
京公网安备 11010802027423号