当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Per-residue Program of Multiple Backbone Dihedral Angles of β-Peptoids via Backbone Substitutions
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-01-09 , DOI: 10.1021/jacs.9b10496 Jumpei Morimoto 1 , Jungyeon Kim 1 , Daisuke Kuroda 1, 2 , Satoru Nagatoishi 3 , Kouhei Tsumoto 1, 2, 3 , Shinsuke Sando 1, 2
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-01-09 , DOI: 10.1021/jacs.9b10496 Jumpei Morimoto 1 , Jungyeon Kim 1 , Daisuke Kuroda 1, 2 , Satoru Nagatoishi 3 , Kouhei Tsumoto 1, 2, 3 , Shinsuke Sando 1, 2
Affiliation
|
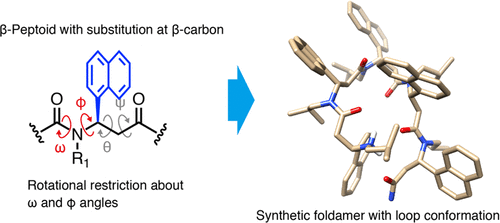
Unique folded structures of natural and synthetic oligomers are the most fundamental basis for their unique functions. N-substituted β-peptides, or β-peptoids, are synthetic oligomers of great potential to fold into diverse three-dimensional structures because of the existence of four rotatable bonds in a monomer and its highly modular synthetic accessibility. However, the existence of the four rotatable bonds poses a challenge for conformational control of β-peptoids. Here, we report a strategy for per-residue programming of two dihedral angles of β-peptoids, which is useful for restricting conformational space of the oligomers. The oligomer was found to form a unique loop conformation that is stabilized by the backbone rotational restrictions. Circular dichroism and NMR spectroscopic analyses and X-ray crystallographic analysis of the oligomer are presented. The strategy would significantly facilitate the discovery of many more unique folded structures of β-peptoids.
中文翻译:

通过骨架取代的β-拟肽的多个骨架二面角的每个残基程序
天然和合成低聚物的独特折叠结构是其独特功能的最基本基础。N-取代的 β-肽或 β-类肽是合成低聚物,具有折叠成不同三维结构的巨大潜力,因为单体中存在四个可旋转的键,并且具有高度模块化的合成可访问性。然而,四个可旋转键的存在对β-拟肽的构象控制提出了挑战。在这里,我们报告了 β-peptoids 的两个二面角的每个残基编程的策略,这对于限制寡聚体的构象空间很有用。发现低聚物形成独特的环构象,该构象通过骨架旋转限制而稳定。给出了低聚物的圆二色性和 NMR 光谱分析和 X 射线晶体学分析。该策略将显着促进发现许多更独特的 β-拟肽折叠结构。
更新日期:2020-01-09
中文翻译:

通过骨架取代的β-拟肽的多个骨架二面角的每个残基程序
天然和合成低聚物的独特折叠结构是其独特功能的最基本基础。N-取代的 β-肽或 β-类肽是合成低聚物,具有折叠成不同三维结构的巨大潜力,因为单体中存在四个可旋转的键,并且具有高度模块化的合成可访问性。然而,四个可旋转键的存在对β-拟肽的构象控制提出了挑战。在这里,我们报告了 β-peptoids 的两个二面角的每个残基编程的策略,这对于限制寡聚体的构象空间很有用。发现低聚物形成独特的环构象,该构象通过骨架旋转限制而稳定。给出了低聚物的圆二色性和 NMR 光谱分析和 X 射线晶体学分析。该策略将显着促进发现许多更独特的 β-拟肽折叠结构。

























 京公网安备 11010802027423号
京公网安备 11010802027423号