当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nanomechanics of Anion-π Interaction in Aqueous Solution
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-01-10 , DOI: 10.1021/jacs.9b11552 Jiawen Zhang 1 , Li Xiang 1 , Bin Yan 1, 2 , Hongbo Zeng 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-01-10 , DOI: 10.1021/jacs.9b11552 Jiawen Zhang 1 , Li Xiang 1 , Bin Yan 1, 2 , Hongbo Zeng 1
Affiliation
|
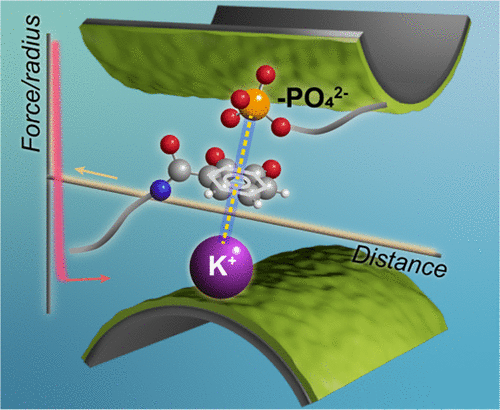
Noncovalent interactions play a constitutive role in numerous biological processes, including biomolecular adhesion, recog-nition and transport. Unlike π-π and cation-π interactions, anion-π interaction was only recently and computationally recognized to participate in biomolecular science. Here, we present the first experimental identification and direct quanti-fication of the nanomechanics of anion-π interaction in aque-ous solution by using a surface forces apparatus with com-plementary computational simulations. Anionic phosphate ester and π-conjugated catecholic moieties that abound in marine bioadhesives were used as the model system. Robust adhesion was observed and attributed to anion-π interaction, which was found to enabled by the cooperative cation-π inter-action due to co-existence of cation. The anion-π interaction strength follows the trend: phosphate ester > HPO42- > SO42-> NO3-, affected by charge density, polarity and hydration effect.
中文翻译:

阴离子-π相互作用在水溶液中的纳米力学
非共价相互作用在许多生物过程中起着组成作用,包括生物分子粘附、识别和运输。与 π-π 和阳离子-π 相互作用不同,阴离子-π 相互作用直到最近才被计算认可参与生物分子科学。在这里,我们通过使用具有互补计算模拟的表面力装置,首次对水溶液中阴离子-π 相互作用的纳米力学进行了实验鉴定和直接量化。海洋生物粘合剂中丰富的阴离子磷酸酯和 π 共轭儿茶酚部分被用作模型系统。观察到牢固的粘附并归因于阴离子-π 相互作用,发现由于阳离子共存而导致协同阳离子-π 相互作用。
更新日期:2020-01-10
中文翻译:

阴离子-π相互作用在水溶液中的纳米力学
非共价相互作用在许多生物过程中起着组成作用,包括生物分子粘附、识别和运输。与 π-π 和阳离子-π 相互作用不同,阴离子-π 相互作用直到最近才被计算认可参与生物分子科学。在这里,我们通过使用具有互补计算模拟的表面力装置,首次对水溶液中阴离子-π 相互作用的纳米力学进行了实验鉴定和直接量化。海洋生物粘合剂中丰富的阴离子磷酸酯和 π 共轭儿茶酚部分被用作模型系统。观察到牢固的粘附并归因于阴离子-π 相互作用,发现由于阳离子共存而导致协同阳离子-π 相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号