当前位置:
X-MOL 学术
›
Commun. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structures of β-glycosidase LXYL-P1-2 reveals the product binding state of GH3 family and a specific pocket for Taxol recognition.
Communications Biology ( IF 5.9 ) Pub Date : 2020-01-10 , DOI: 10.1038/s42003-019-0744-4 Lin Yang 1 , Tian-Jiao Chen 2 , Fen Wang 2 , Li Li 2 , Wen-Bo Yu 2 , Yi-Kang Si 2 , Jing-Jing Chen 2 , Wan-Cang Liu 2 , Ping Zhu 2 , Weimin Gong 1
Communications Biology ( IF 5.9 ) Pub Date : 2020-01-10 , DOI: 10.1038/s42003-019-0744-4 Lin Yang 1 , Tian-Jiao Chen 2 , Fen Wang 2 , Li Li 2 , Wen-Bo Yu 2 , Yi-Kang Si 2 , Jing-Jing Chen 2 , Wan-Cang Liu 2 , Ping Zhu 2 , Weimin Gong 1
Affiliation
|
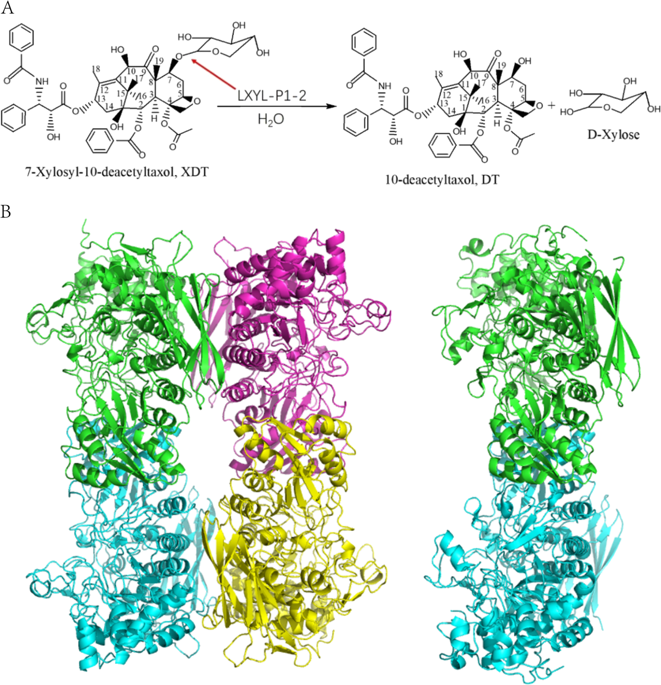
LXYL-P1-2 is one of the few xylosidases that efficiently catalyze the reaction from 7-β-xylosyl-10-deacetyltaxol (XDT) to 10-deacetyltaxol (DT), and is a potential enzyme used in Taxol industrial production. Here we report the crystal structure of LXYL-P1-2 and its XDT binding complex. These structures reveal an enzyme/product complex with the sugar conformation different from the enzyme/substrate complex reported previously in GH3 enzymes, even in the whole glycohydrolases family. In addition, the DT binding pocket is identified as the structural basis for the substrate specificity. Further structure analysis reveals common features in LXYL-P1-2 and Taxol binding protein tubulin, which might provide useful information for designing new Taxol carrier proteins for drug delivery.
中文翻译:

β-糖苷酶LXYL-P1-2的结构揭示了GH3家族的产物结合状态和紫杉醇识别的特定口袋。
LXYL-P1-2是能有效催化从7-β-木糖基-10-去乙酰紫杉醇(XDT)到10-去乙酰基紫杉醇(DT)的反应的少数木糖苷酶之一,并且是在紫杉醇工业生产中使用的潜在酶。在这里,我们报告LXYL-P1-2的晶体结构及其XDT结合复合物。这些结构揭示了具有糖构象的酶/产物复合物,其与先前在GH3酶中报道的酶/底物复合物不同,甚至在整个糖水解酶家族中也是如此。另外,DT结合袋被确定为底物特异性的结构基础。进一步的结构分析揭示了LXYL-P1-2和紫杉醇结合蛋白微管蛋白的共同特征,这可能为设计用于药物递送的新紫杉醇载体蛋白提供有用的信息。
更新日期:2020-01-10
中文翻译:

β-糖苷酶LXYL-P1-2的结构揭示了GH3家族的产物结合状态和紫杉醇识别的特定口袋。
LXYL-P1-2是能有效催化从7-β-木糖基-10-去乙酰紫杉醇(XDT)到10-去乙酰基紫杉醇(DT)的反应的少数木糖苷酶之一,并且是在紫杉醇工业生产中使用的潜在酶。在这里,我们报告LXYL-P1-2的晶体结构及其XDT结合复合物。这些结构揭示了具有糖构象的酶/产物复合物,其与先前在GH3酶中报道的酶/底物复合物不同,甚至在整个糖水解酶家族中也是如此。另外,DT结合袋被确定为底物特异性的结构基础。进一步的结构分析揭示了LXYL-P1-2和紫杉醇结合蛋白微管蛋白的共同特征,这可能为设计用于药物递送的新紫杉醇载体蛋白提供有用的信息。



























 京公网安备 11010802027423号
京公网安备 11010802027423号