当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Measurement and Correlation of Solubility of Benzothiazolium Ionic Liquids in Ethanol + Ethyl Benzoate
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.jced.9b00997 Tian Yao 1 , Kaifeng Du 2
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.jced.9b00997 Tian Yao 1 , Kaifeng Du 2
Affiliation
|
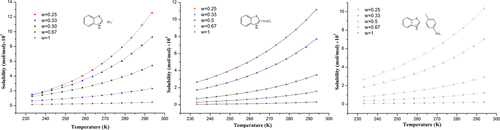
The solubilities of benzothazolium-based ionic liquids (ILs) [HBth][BF4], [HBth][CH3SO3], and [HBth][p-TSA] in binary ethanol + ethyl benzoate solvents were measured using the equilibrium method with the temperature range from 233.2 to 293.7 K at atmospheric pressure. Results of these measurements were correlated by both λh equation and the modified Apelblat equation. It was found that both models gave satisfactory correlation results. In the λh model, the average relative deviations were 0.7145, 0.7845, and 0.8119% for [HBth][BF4], [HBth][CH3SO3], and [HBth][p-TSA], respectively. Interestingly, the parameters of λ and h for the λh model and A, B, and C for the modified Apelblat model were simultaneously expressed as functions of solvent composition (xsol) for the first time. Furthermore, solubilities of these three ILs in the binary system were predicted through these two sets of self-created correlation equations with ideal performances. The total average relative deviations were less than 2.2% for both models. These successful in-depth correlations and validated prediction results have guaranteed the accuracy of experimental solubility data and provide us a reliable method for accurate solubility prediction at any temperature with any solvent composition. This developed calculation method in this report lays a foundation for accurate industrial recovery of ILs and gives us valuable guidance for the design of the specific purification process to acquire desired purity of the product in industrial production.
中文翻译:

苯并噻唑鎓离子液体在乙醇+苯甲酸乙酯中的溶解度的测量和相关性
使用平衡法测量苯并噻唑基离子液体(ILs)[HBth] [BF 4 ],[HBth] [CH 3 SO 3 ]和[HBth] [ p -TSA]在二元乙醇+苯甲酸乙酯溶剂中的溶解度在大气压下,温度范围为233.2至293.7K。这些测量的结果由两个λ相关ħ方程和改性Apelblat方程。发现两个模型都给出了令人满意的相关结果。在λ ħ模型,平均相对偏差分别为[HBth] [BF 0.7145,0.7845和0.8119%4 ],[HBth] [CH 3 SO 3 ]和[HBth] [ p-TSA]。有趣的是,λ和参数ħ为λ ħ模型和甲,乙,和Ç对于修改Apelblat模型中同时表示为溶剂组合物的函数(X溶胶)。此外,通过这两组具有理想性能的自相关方程,可以预测这三个IL在二元系统中的溶解度。两个模型的总平均相对偏差均小于2.2%。这些成功的深度关联和有效的预测结果确保了实验溶解度数据的准确性,并为我们提供了一种在任何温度下以任何溶剂组成准确预测溶解度的可靠方法。本报告中这种发达的计算方法为精确地回收白介素奠定了基础,并为我们设计特定的纯化工艺以在工业生产中获得所需纯度的产品提供了宝贵的指导。
更新日期:2020-01-10
中文翻译:

苯并噻唑鎓离子液体在乙醇+苯甲酸乙酯中的溶解度的测量和相关性
使用平衡法测量苯并噻唑基离子液体(ILs)[HBth] [BF 4 ],[HBth] [CH 3 SO 3 ]和[HBth] [ p -TSA]在二元乙醇+苯甲酸乙酯溶剂中的溶解度在大气压下,温度范围为233.2至293.7K。这些测量的结果由两个λ相关ħ方程和改性Apelblat方程。发现两个模型都给出了令人满意的相关结果。在λ ħ模型,平均相对偏差分别为[HBth] [BF 0.7145,0.7845和0.8119%4 ],[HBth] [CH 3 SO 3 ]和[HBth] [ p-TSA]。有趣的是,λ和参数ħ为λ ħ模型和甲,乙,和Ç对于修改Apelblat模型中同时表示为溶剂组合物的函数(X溶胶)。此外,通过这两组具有理想性能的自相关方程,可以预测这三个IL在二元系统中的溶解度。两个模型的总平均相对偏差均小于2.2%。这些成功的深度关联和有效的预测结果确保了实验溶解度数据的准确性,并为我们提供了一种在任何温度下以任何溶剂组成准确预测溶解度的可靠方法。本报告中这种发达的计算方法为精确地回收白介素奠定了基础,并为我们设计特定的纯化工艺以在工业生产中获得所需纯度的产品提供了宝贵的指导。

























 京公网安备 11010802027423号
京公网安备 11010802027423号