当前位置:
X-MOL 学术
›
Nat. Struct. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Defining the landscape of ATP-competitive inhibitor resistance residues in protein kinases.
Nature Structural & Molecular Biology ( IF 16.8 ) Pub Date : 2020-01-10 , DOI: 10.1038/s41594-019-0358-z N S Persky 1 , D Hernandez 1 , M Do Carmo 1 , L Brenan 1 , O Cohen 1, 2, 3, 4 , S Kitajima 2 , U Nayar 1, 2, 3, 4 , A Walker 1 , S Pantel 1 , Y Lee 1 , J Cordova 5 , M Sathappa 1 , C Zhu 1 , T K Hayes 1, 2 , P Ram 1, 2, 3, 4 , P Pancholi 2 , T S Mikkelsen 1, 6, 7 , D A Barbie 2 , X Yang 1 , R Haq 2 , F Piccioni 1 , D E Root 1 , C M Johannessen 1, 8
Nature Structural & Molecular Biology ( IF 16.8 ) Pub Date : 2020-01-10 , DOI: 10.1038/s41594-019-0358-z N S Persky 1 , D Hernandez 1 , M Do Carmo 1 , L Brenan 1 , O Cohen 1, 2, 3, 4 , S Kitajima 2 , U Nayar 1, 2, 3, 4 , A Walker 1 , S Pantel 1 , Y Lee 1 , J Cordova 5 , M Sathappa 1 , C Zhu 1 , T K Hayes 1, 2 , P Ram 1, 2, 3, 4 , P Pancholi 2 , T S Mikkelsen 1, 6, 7 , D A Barbie 2 , X Yang 1 , R Haq 2 , F Piccioni 1 , D E Root 1 , C M Johannessen 1, 8
Affiliation
|
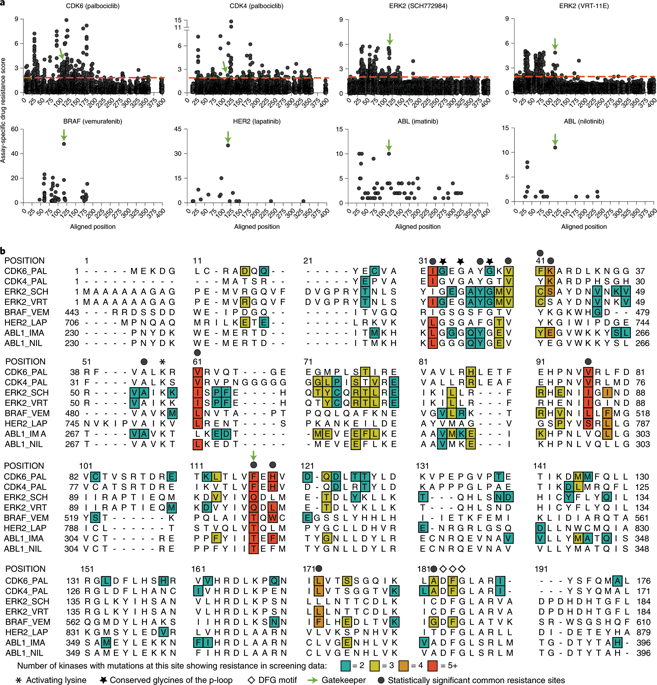
Kinases are involved in disease development and modulation of their activity can be therapeutically beneficial. Drug-resistant mutant kinases are valuable tools in drug discovery efforts, but the prediction of mutants across the kinome is challenging. Here, we generate deep mutational scanning data to identify mutant mammalian kinases that drive resistance to clinically relevant inhibitors. We aggregate these data with subsaturation mutagenesis data and use it to develop, test and validate a framework to prospectively identify residues that mediate kinase activity and drug resistance across the kinome. We validate predicted resistance mutations in CDK4, CDK6, ERK2, EGFR and HER2. Capitalizing on a highly predictable residue, we generate resistance mutations in TBK1, CSNK2A1 and BRAF. Unexpectedly, we uncover a potentially generalizable activation site that mediates drug resistance and confirm its impact in BRAF, EGFR, HER2 and MEK1. We anticipate that the identification of these residues will enable the broad interrogation of the kinome and its inhibitors.
中文翻译:

定义蛋白激酶中ATP竞争性抑制剂耐药性残基的态势。
激酶参与疾病的发展,其活性的调节可以在治疗上有益。耐药突变激酶是药物发现工作中的宝贵工具,但是预测整个kinome突变体具有挑战性。在这里,我们生成了深层的突变扫描数据,以识别出对临床相关抑制剂具有耐药性的突变型哺乳动物激酶。我们将这些数据与亚饱和诱变数据进行汇总,并使用它来开发,测试和验证一个框架,以前瞻性地识别介导激酶活性和整个激酶组耐药性的残基。我们验证了CDK4,CDK6,ERK2,EGFR和HER2中预测的耐药性突变。利用高度可预测的残基,我们在TBK1,CSNK2A1和BRAF中产生抗性突变。不料,我们发现了可能介导的耐药性激活位点,并证实了其对BRAF,EGFR,HER2和MEK1的影响。我们预计,鉴定这些残基将使人们能够广泛地研究激酶组及其抑制剂。
更新日期:2020-01-10
中文翻译:

定义蛋白激酶中ATP竞争性抑制剂耐药性残基的态势。
激酶参与疾病的发展,其活性的调节可以在治疗上有益。耐药突变激酶是药物发现工作中的宝贵工具,但是预测整个kinome突变体具有挑战性。在这里,我们生成了深层的突变扫描数据,以识别出对临床相关抑制剂具有耐药性的突变型哺乳动物激酶。我们将这些数据与亚饱和诱变数据进行汇总,并使用它来开发,测试和验证一个框架,以前瞻性地识别介导激酶活性和整个激酶组耐药性的残基。我们验证了CDK4,CDK6,ERK2,EGFR和HER2中预测的耐药性突变。利用高度可预测的残基,我们在TBK1,CSNK2A1和BRAF中产生抗性突变。不料,我们发现了可能介导的耐药性激活位点,并证实了其对BRAF,EGFR,HER2和MEK1的影响。我们预计,鉴定这些残基将使人们能够广泛地研究激酶组及其抑制剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号