当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phosphine boranes as less hydrophobic building blocks than alkanes and silanes: Structure-property relationship and estrogen-receptor-modulating potency of 4-phosphinophenol derivatives.
Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2020-01-09 , DOI: 10.1016/j.bmc.2020.115310 Hiroki Saito 1 , Yuichiro Matsumoto 1 , Yuichi Hashimoto 1 , Shinya Fujii 2
Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2020-01-09 , DOI: 10.1016/j.bmc.2020.115310 Hiroki Saito 1 , Yuichiro Matsumoto 1 , Yuichi Hashimoto 1 , Shinya Fujii 2
Affiliation
|
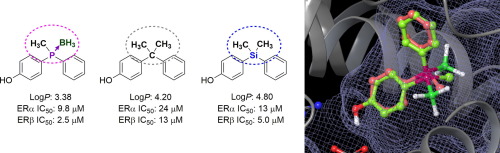
Increasing structural options in medicinal chemistry is important for the development of novel and distinctive drug candidates. In this study, we focused on phosphorus-containing functionalities. We designed and synthesized a series of phosphinophenol derivatives and determined their physicochemical properties, including hydrophobicity parameter LogP, and their biological activity toward estrogen receptor (ER). Notably, the phosphine borane derivatives (9 and 14) exhibited potent ER-antagonistic activity, exceeding the potency of the corresponding alkane (15) and silane (16) derivatives, despite having a less hydrophobic nature. The determined physicochemical parameters will be helpful for the rational design of phosphorus-containing biologically active compounds. Our results indicate that phosphine boranes are a promising new chemical entry in the range of structural options for drug discovery.
中文翻译:

与烷烃和硅烷相比,膦硼烷的疏水性构建基少:4-膦基苯酚衍生物的结构-性质关系和雌激素-受体调节潜能。
药物化学中越来越多的结构选择对于开发新颖独特的候选药物很重要。在这项研究中,我们集中于含磷的功能。我们设计和合成了一系列膦基酚衍生物,并确定了其理化性质,包括疏水性参数LogP,以及它们对雌激素受体(ER)的生物活性。值得注意的是,尽管具有较少的疏水性,但是膦硼烷衍生物(9和14)显示出强的ER拮抗活性,超过了相应的烷烃(15)和硅烷(16)衍生物的效力。确定的理化参数将有助于合理设计含磷生物活性化合物。
更新日期:2020-01-10
中文翻译:

与烷烃和硅烷相比,膦硼烷的疏水性构建基少:4-膦基苯酚衍生物的结构-性质关系和雌激素-受体调节潜能。
药物化学中越来越多的结构选择对于开发新颖独特的候选药物很重要。在这项研究中,我们集中于含磷的功能。我们设计和合成了一系列膦基酚衍生物,并确定了其理化性质,包括疏水性参数LogP,以及它们对雌激素受体(ER)的生物活性。值得注意的是,尽管具有较少的疏水性,但是膦硼烷衍生物(9和14)显示出强的ER拮抗活性,超过了相应的烷烃(15)和硅烷(16)衍生物的效力。确定的理化参数将有助于合理设计含磷生物活性化合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号