Journal of Fluorine Chemistry ( IF 1.9 ) Pub Date : 2020-01-10 , DOI: 10.1016/j.jfluchem.2020.109452 Kohei Fuchibe , Masashi Abe , Mizore Sasaki , Junji Ichikawa
|
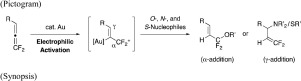
Transition metal-catalyzed nucleophilic addition of heteroatom nucleophiles to 1,1-difluoroallenes has been demonstrated here for the first time. Treatment of 1,1-difluoroallenes with O-nucleophiles, such as phenols, carboxylic acids, and sulfonic acids, facilitated unprecedented α-selective addition in the presence of AuCl3 or AuCl(PPh3)/AgSbF6 catalysts which afforded 1,1-difluoroallyl alcohol derivatives. In contrast, amides and thiols underwent γ-selective addition to 1,1-difluoroallenes on their nitrogen or sulfur centers to provide 3,3-difluoroallyl amine and thiol derivatives.
中文翻译:

金催化的1,1-二氟丙二烯的亲电子活化:杂原子亲核试剂的α和γ选择性加成
此处首次证明了过渡金属催化的杂原子亲核试剂向1,1-二氟丙二烯的亲核加成。在AuCl 3或AuCl(PPh 3)/ AgSbF 6催化剂存在下,用O-亲核试剂(如苯酚,羧酸和磺酸)处理1,1-二氟丙二烯促进了前所未有的α-选择性加成反应,得到1,1 -二氟烯丙基醇衍生物。相反,酰胺和硫醇在它们的氮或硫中心上对1,1-二氟丙二烯进行γ-选择性加成以提供3,3-二氟烯丙基胺和硫醇衍生物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号