当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Oxygen vacancy mediated surface charge redistribution of Cu-substituted LaFeO3 for degradation of bisphenol A by efficient decomposition of H2O2.
Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2020-01-10 , DOI: 10.1016/j.jhazmat.2020.122072 Keliang Pan 1 , Changzhu Yang 1 , Jingping Hu 1 , Wenlong Yang 1 , Bingchuan Liu 1 , Jiakuan Yang 2 , Sha Liang 1 , Keke Xiao 1 , Huijie Hou 1
Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2020-01-10 , DOI: 10.1016/j.jhazmat.2020.122072 Keliang Pan 1 , Changzhu Yang 1 , Jingping Hu 1 , Wenlong Yang 1 , Bingchuan Liu 1 , Jiakuan Yang 2 , Sha Liang 1 , Keke Xiao 1 , Huijie Hou 1
Affiliation
|
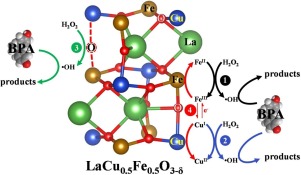
The novel catalyst LaCu0.5Fe0.5O3-δ with oxygen vacancies (OVs) was prepared and demonstrated excellent stability and activity for the degradation of bisphenol A. The removal rate of 92.1 % and H2O2 utilization efficiency of 70.4 % were obtained due to the efficient hydroxyl radical generation mediated by OVs. The density functional theory calculation showed that the substitution of Cu and formation of OVs significantly increases the charge density near the active sites. Bader charge analysis revealed that the charge offset accelerated the reduction of Fe. The elevation of electron transfer efficiency also promotes the valence transition of copper and iron atoms. The reversible electronic transition between Fe2+ ⇆ Fe3+, Cu+ ⇆ Cu2+ and Cu2+ ⇆ Fe2+ involved in this reaction were considered to be enhanced and the homolytic bond clearage of H2O2 was simultaneously promoted, facilitated by the electron-rich region combined with OVs on the surface of LaCu0.5Fe0.5O3-δ.
中文翻译:

氧空位介导的铜取代的LaFeO3的表面电荷再分布,可通过H2O2的有效分解来降解双酚A。
制备了具有氧空位(OVs)的新型催化剂LaCu0.5Fe0.5O3-δ,并证明了其对双酚A降解的优异稳定性和活性。由于其高效,获得了92.1%的去除率和70.4%的H2O2利用率。 OVs介导的羟基自由基的产生。密度泛函理论计算表明,Cu的取代和OV的形成显着增加了活性位附近的电荷密度。Bader电荷分析表明,电荷抵消加速了Fe的还原。电子转移效率的提高还促进了铜和铁原子的化合价跃迁。Fe2 +⇆Fe3 +之间的可逆电子跃迁,
更新日期:2020-01-11
中文翻译:

氧空位介导的铜取代的LaFeO3的表面电荷再分布,可通过H2O2的有效分解来降解双酚A。
制备了具有氧空位(OVs)的新型催化剂LaCu0.5Fe0.5O3-δ,并证明了其对双酚A降解的优异稳定性和活性。由于其高效,获得了92.1%的去除率和70.4%的H2O2利用率。 OVs介导的羟基自由基的产生。密度泛函理论计算表明,Cu的取代和OV的形成显着增加了活性位附近的电荷密度。Bader电荷分析表明,电荷抵消加速了Fe的还原。电子转移效率的提高还促进了铜和铁原子的化合价跃迁。Fe2 +⇆Fe3 +之间的可逆电子跃迁,


























 京公网安备 11010802027423号
京公网安备 11010802027423号