当前位置:
X-MOL 学术
›
Exp. Cell Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
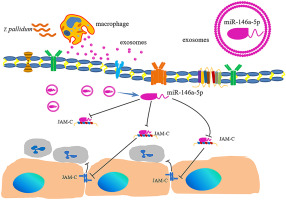
Exosomal miR-146a-5p from Treponema pallidum-stimulated macrophages reduces endothelial cells permeability and monocyte transendothelial migration by targeting JAM-C.
Experimental Cell Research ( IF 3.7 ) Pub Date : 2020-01-09 , DOI: 10.1016/j.yexcr.2020.111823 Wenlong Hu 1 , Bufang Xu 2 , Jingping Zhang 2 , Caixia Kou 2 , Jinquan Liu 2 , Qianqiu Wang 2 , Ruili Zhang 3
Experimental Cell Research ( IF 3.7 ) Pub Date : 2020-01-09 , DOI: 10.1016/j.yexcr.2020.111823 Wenlong Hu 1 , Bufang Xu 2 , Jingping Zhang 2 , Caixia Kou 2 , Jinquan Liu 2 , Qianqiu Wang 2 , Ruili Zhang 3
Affiliation
|
Exosomal microRNAs (miRNAs) transferred between cells have been implicated in modulating the host immune response in microbial infections. In this study, we isolated exosomes from Treponema pallidum (T. pallidum)-stimulated macrophages and detected differential exosomal miRNA expression using both microarrays, and RT-qPCR. A total of 65 differentially expressed miRNAs (35 upregulated and 30 downregulated) were identified. Of all identified miRNAs, miR-146a-5p was one of the most significantly changed miRNAs with high expression in exosomes from T. pallidum-stimulated macrophages. Furthermore, we isolated plasma exosomes from early syphilis patients and healthy controls, and confirmed miR-146a-5p upregulation in the former group. We also show that exosomal miR-146a-5p is efficiently transported into endothelial cells, reducing monocyte transendothelial migration and endothelial permeability by targeting junctional adhesion molecule C (JAM-C). Luciferase reporter assays confirmed binding of exosomal miR-146a-5p to the 3'untranslated region (3'UTR) of JAM-C. We then demonstrated that also exosomes derived from macrophages stimulated by T. pallidum expressed high levels of miR-146a-5p which could be delivered to endothelial cells, and decreased monocyte transendothelial migration by targeting JAM-C. Overall, this work provides novel insights into the mechanism by which T. pallidum hampers inflammatory reactions of the host via a blockade of leukocytes transendothelial migration and endothelial permeability.
中文翻译:

梅毒螺旋体刺激巨噬细胞的外泌体miR-146a-5p通过靶向JAM-C降低内皮细胞通透性和单核细胞跨内皮迁移。
细胞之间转移的外泌体微小RNA(miRNA)与调节微生物感染中的宿主免疫反应有关。在这项研究中,我们从梅毒螺旋体(T. pallidum)刺激的巨噬细胞中分离了外泌体,并使用微阵列和RT-qPCR检测了不同的外泌体miRNA表达。总共鉴定出65个差异表达的miRNA(35个上调和30个下调)。在所有已鉴定的miRNA中,miR-146a-5p是变化最明显的miRNA之一,在苍白球刺激的巨噬细胞的外泌体中高表达。此外,我们从早期梅毒患者和健康对照中分离出血浆外泌体,并确认前一组中miR-146a-5p上调。我们还显示外泌体miR-146a-5p可有效转运到内皮细胞中,通过靶向连接黏附分子C(JAM-C)减少单核细胞跨内皮迁移和内皮通透性。萤光素酶报告基因测定证实了外泌体miR-146a-5p与JAM-C的3'非翻译区(3'UTR)结合。然后,我们证明了由苍白螺旋体刺激的巨噬细胞衍生的外泌体也表达了高水平的miR-146a-5p,可以将其递送至内皮细胞,并通过靶向JAM-C减少单核细胞的跨内皮迁移。总体而言,这项工作提供了新的见解,以了解苍白螺旋体通过阻断白细胞跨内皮迁移和内皮通透性而阻碍宿主的炎症反应。萤光素酶报告基因测定证实了外泌体miR-146a-5p与JAM-C的3'非翻译区(3'UTR)结合。然后,我们证明了由苍白螺旋体刺激的巨噬细胞衍生的外泌体也表达了高水平的miR-146a-5p,可以将其递送至内皮细胞,并通过靶向JAM-C减少单核细胞的跨内皮迁移。总体而言,这项工作提供了新的见解,以了解苍白螺旋体通过阻断白细胞跨内皮迁移和内皮通透性而阻碍宿主的炎症反应。萤光素酶报告基因测定证实了外泌体miR-146a-5p与JAM-C的3'非翻译区(3'UTR)结合。然后,我们证明了由苍白螺旋体刺激的巨噬细胞衍生的外泌体也表达了高水平的miR-146a-5p,可以将其递送至内皮细胞,并通过靶向JAM-C减少单核细胞的跨内皮迁移。总体而言,这项工作提供了新的见解,以了解苍白螺旋体通过阻断白细胞跨内皮迁移和内皮通透性而阻碍宿主的炎症反应。靶向JAM-C可减少单核细胞跨内皮迁移。总的来说,这项工作提供了新的见解,以了解苍白螺旋体通过阻断白细胞跨内皮迁移和内皮通透性而阻碍宿主的炎症反应。靶向JAM-C可减少单核细胞跨内皮迁移。总体而言,这项工作提供了新的见解,以了解苍白螺旋体通过阻断白细胞跨内皮迁移和内皮通透性而阻碍宿主的炎症反应。
更新日期:2020-01-09
中文翻译:

梅毒螺旋体刺激巨噬细胞的外泌体miR-146a-5p通过靶向JAM-C降低内皮细胞通透性和单核细胞跨内皮迁移。
细胞之间转移的外泌体微小RNA(miRNA)与调节微生物感染中的宿主免疫反应有关。在这项研究中,我们从梅毒螺旋体(T. pallidum)刺激的巨噬细胞中分离了外泌体,并使用微阵列和RT-qPCR检测了不同的外泌体miRNA表达。总共鉴定出65个差异表达的miRNA(35个上调和30个下调)。在所有已鉴定的miRNA中,miR-146a-5p是变化最明显的miRNA之一,在苍白球刺激的巨噬细胞的外泌体中高表达。此外,我们从早期梅毒患者和健康对照中分离出血浆外泌体,并确认前一组中miR-146a-5p上调。我们还显示外泌体miR-146a-5p可有效转运到内皮细胞中,通过靶向连接黏附分子C(JAM-C)减少单核细胞跨内皮迁移和内皮通透性。萤光素酶报告基因测定证实了外泌体miR-146a-5p与JAM-C的3'非翻译区(3'UTR)结合。然后,我们证明了由苍白螺旋体刺激的巨噬细胞衍生的外泌体也表达了高水平的miR-146a-5p,可以将其递送至内皮细胞,并通过靶向JAM-C减少单核细胞的跨内皮迁移。总体而言,这项工作提供了新的见解,以了解苍白螺旋体通过阻断白细胞跨内皮迁移和内皮通透性而阻碍宿主的炎症反应。萤光素酶报告基因测定证实了外泌体miR-146a-5p与JAM-C的3'非翻译区(3'UTR)结合。然后,我们证明了由苍白螺旋体刺激的巨噬细胞衍生的外泌体也表达了高水平的miR-146a-5p,可以将其递送至内皮细胞,并通过靶向JAM-C减少单核细胞的跨内皮迁移。总体而言,这项工作提供了新的见解,以了解苍白螺旋体通过阻断白细胞跨内皮迁移和内皮通透性而阻碍宿主的炎症反应。萤光素酶报告基因测定证实了外泌体miR-146a-5p与JAM-C的3'非翻译区(3'UTR)结合。然后,我们证明了由苍白螺旋体刺激的巨噬细胞衍生的外泌体也表达了高水平的miR-146a-5p,可以将其递送至内皮细胞,并通过靶向JAM-C减少单核细胞的跨内皮迁移。总体而言,这项工作提供了新的见解,以了解苍白螺旋体通过阻断白细胞跨内皮迁移和内皮通透性而阻碍宿主的炎症反应。靶向JAM-C可减少单核细胞跨内皮迁移。总的来说,这项工作提供了新的见解,以了解苍白螺旋体通过阻断白细胞跨内皮迁移和内皮通透性而阻碍宿主的炎症反应。靶向JAM-C可减少单核细胞跨内皮迁移。总体而言,这项工作提供了新的见解,以了解苍白螺旋体通过阻断白细胞跨内皮迁移和内皮通透性而阻碍宿主的炎症反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号