当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Measurement and correlation of thermophysical properties in aqueous solutions of some novel bio-based deep eutectic solvents (lactic acid/amino acids) at T = (298.15 to 313.15) K
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jct.2020.106051 Masumeh Mokhtarpour , Hemayat Shekaari
The Journal of Chemical Thermodynamics ( IF 2.6 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.jct.2020.106051 Masumeh Mokhtarpour , Hemayat Shekaari
|
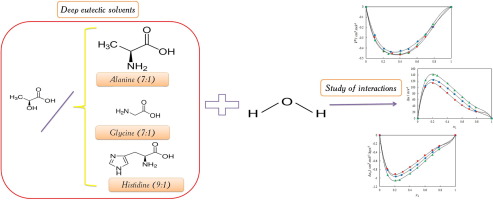
Abstract Thermodynamic properties of novel bio-based deep eutectic solvents (DESs) and their mixture with other solvents is of great importance in extraction and pharmaceutical sciences. In the present study new class of these neoteric sustainable solvents composed of amino acids and lactic acid have been prepared, density and speed of sound data for neat lactic acid/alanine, lactic acid/glycine and lactic acid/histidine and their aqueous mixture have been measured over the entire composition range at T = (298.15, 303.15, 308.15 and 313.15) K. Based on these data, some of the thermodynamic properties including the excess molar volumes (VE), apparent molar volume ( V ϕ ), excess isobaric thermal expansion ( α p , m E ), the excess molar isentropic compressibility (κEs,m) and limiting apparent molar expansibility ( ϕ E , 1 0 ) have been calculated. For all of the studied systems above, these important properties have been analysed based on various solute-solvent interactions, occurring between the components of the mixtures. Furthermore, it has been found that all the investigated DESs act as a structure-breaker in water over the whole temperature range using Hepler’s constant. The properties have been correlated by the Redlich-Kister type equations.
中文翻译:

在 T = (298.15 to 313.15) K 时一些新型生物基深共熔溶剂(乳酸/氨基酸)水溶液中热物理性质的测量和相关性
摘要 新型生物基深共熔溶剂 (DESs) 及其与其他溶剂的混合物的热力学性质在提取和制药科学中具有重要意义。在本研究中,制备了由氨基酸和乳酸组成的新型可持续溶剂,纯乳酸/丙氨酸、乳酸/甘氨酸和乳酸/组氨酸及其水性混合物的密度和声速数据已得到在 T = (298.15, 303.15, 308.15 和 313.15) K 的整个组成范围内测量。基于这些数据,一些热力学性质包括过量摩尔体积 (VE)、表观摩尔体积 (V φ )、过量等压热膨胀 ( α p , m E )、过量摩尔等熵压缩率 (κEs,m) 和极限表观摩尔膨胀率 ( ϕ E , 1 0 ) 已经计算出来。对于上述所有研究的系统,这些重要的特性已根据混合物组分之间发生的各种溶质 - 溶剂相互作用进行了分析。此外,已经发现所有研究的 DES 在使用 Hepler 常数的整个温度范围内都充当水中的结构破坏剂。这些属性已通过 Redlich-Kister 类型方程相关联。
更新日期:2020-05-01
中文翻译:

在 T = (298.15 to 313.15) K 时一些新型生物基深共熔溶剂(乳酸/氨基酸)水溶液中热物理性质的测量和相关性
摘要 新型生物基深共熔溶剂 (DESs) 及其与其他溶剂的混合物的热力学性质在提取和制药科学中具有重要意义。在本研究中,制备了由氨基酸和乳酸组成的新型可持续溶剂,纯乳酸/丙氨酸、乳酸/甘氨酸和乳酸/组氨酸及其水性混合物的密度和声速数据已得到在 T = (298.15, 303.15, 308.15 和 313.15) K 的整个组成范围内测量。基于这些数据,一些热力学性质包括过量摩尔体积 (VE)、表观摩尔体积 (V φ )、过量等压热膨胀 ( α p , m E )、过量摩尔等熵压缩率 (κEs,m) 和极限表观摩尔膨胀率 ( ϕ E , 1 0 ) 已经计算出来。对于上述所有研究的系统,这些重要的特性已根据混合物组分之间发生的各种溶质 - 溶剂相互作用进行了分析。此外,已经发现所有研究的 DES 在使用 Hepler 常数的整个温度范围内都充当水中的结构破坏剂。这些属性已通过 Redlich-Kister 类型方程相关联。


























 京公网安备 11010802027423号
京公网安备 11010802027423号