当前位置:
X-MOL 学术
›
Colloids Surf. A Physicochem. Eng. Aspects
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Aggregation of sodium dodecylbenzene sulfonate: weak molecular interactions modulated by imidazolium cation of short alkyl chain length
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 5.2 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.colsurfa.2020.124435 Álvaro Javier Patiño Agudelo , Guilherme Max Dias Ferreira , Gabriel Max Dias Ferreira , Yara Luiza Coelho , Eliara Acipreste Hudson , Ana Clarissa dos Santos Pires , Luis Henrique Mendes da Silva
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 5.2 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.colsurfa.2020.124435 Álvaro Javier Patiño Agudelo , Guilherme Max Dias Ferreira , Gabriel Max Dias Ferreira , Yara Luiza Coelho , Eliara Acipreste Hudson , Ana Clarissa dos Santos Pires , Luis Henrique Mendes da Silva
|
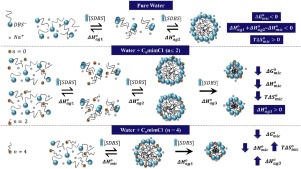
Abstract Ionic liquids (ILs) can modify cooperative process in aqueous solutions to a large extent, including anionic surfactant aggregation. Here, the micellization of sodium dodecylbenzene sulfonate (SDBS) was evaluated in low concentrations of 1-alkyl-3-methylimidazolium chloride (CnmimCl, n = 0, 2, and 4) aqueous solutions through fluorescence spectroscopy, isothermal titration calorimetry, dynamic light scattering, and conductometry. The thermodynamic stability of SDBS aggregates strongly depended on the IL structure and concentration, following the order C4mim+ > C0mim+ ≈ C2mim+. At 1.0 mmol L−1 of the ILs, the increase of the hydrophobicity of the imidazolium cation decreased the enthalpic favorableness, changing Δ H m i c o from −3.75 ± 0.07 kJ mol−1, for C0mim+, to −2.69 ± 0.01 kJ mol−1, for C4mim+. On the other hand, the entropic feasibility showed an opposite trend, i.e., the higher hydrophobicity of C4mim+ overcame the kosmotropic effect of IL cations in the bulks. We suggested that the imidazolium cations interact with the SDBS monomers on the micellar surface, mainly through hydrophobic, π-π, and electrostatic interactions for C4mim+ and C2mim+, and through electrostatic interactions and hydrogen bonds for C0mim+.
中文翻译:

十二烷基苯磺酸钠的聚集:短烷基链长咪唑鎓阳离子调节的弱分子相互作用
摘要 离子液体(ILs)可以在很大程度上改变水溶液中的协同过程,包括阴离子表面活性剂的聚集。在这里,通过荧光光谱法、等温滴定量热法、动态光散射法在低浓度的 1-烷基-3-甲基咪唑氯化物(CnmimCl,n = 0、2 和 4)水溶液中评估了十二烷基苯磺酸钠 (SDBS) 的胶束化,和电导。SDBS 聚集体的热力学稳定性强烈依赖于 IL 结构和浓度,顺序为 C4mim+ > C0mim+ ≈ C2mim+。在 1.0 mmol L-1 ILs 时,咪唑鎓阳离子疏水性的增加降低了焓有利,将 Δ H mico 从 -3.75 ± 0.07 kJ mol-1,对于 C0mim+ 改变为 -2.69 ± 0.01 kJ mol-1 ,对于 C4mim+。另一方面,熵的可行性显示出相反的趋势,即 C4mim+ 的较高疏水性克服了 IL 阳离子在本体中的亲液效应。我们认为咪唑鎓阳离子与胶束表面的 SDBS 单体相互作用,主要通过疏水性、π-π 和 C4mim+ 和 C2mim+ 的静电相互作用,以及 C0mim+ 的静电相互作用和氢键相互作用。
更新日期:2020-02-01
中文翻译:

十二烷基苯磺酸钠的聚集:短烷基链长咪唑鎓阳离子调节的弱分子相互作用
摘要 离子液体(ILs)可以在很大程度上改变水溶液中的协同过程,包括阴离子表面活性剂的聚集。在这里,通过荧光光谱法、等温滴定量热法、动态光散射法在低浓度的 1-烷基-3-甲基咪唑氯化物(CnmimCl,n = 0、2 和 4)水溶液中评估了十二烷基苯磺酸钠 (SDBS) 的胶束化,和电导。SDBS 聚集体的热力学稳定性强烈依赖于 IL 结构和浓度,顺序为 C4mim+ > C0mim+ ≈ C2mim+。在 1.0 mmol L-1 ILs 时,咪唑鎓阳离子疏水性的增加降低了焓有利,将 Δ H mico 从 -3.75 ± 0.07 kJ mol-1,对于 C0mim+ 改变为 -2.69 ± 0.01 kJ mol-1 ,对于 C4mim+。另一方面,熵的可行性显示出相反的趋势,即 C4mim+ 的较高疏水性克服了 IL 阳离子在本体中的亲液效应。我们认为咪唑鎓阳离子与胶束表面的 SDBS 单体相互作用,主要通过疏水性、π-π 和 C4mim+ 和 C2mim+ 的静电相互作用,以及 C0mim+ 的静电相互作用和氢键相互作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号