当前位置:
X-MOL 学术
›
Curr. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Phosphatase PP1 Promotes Mitotic Slippage through Mad3 Dephosphorylation.
Current Biology ( IF 9.2 ) Pub Date : 2020-01-09 , DOI: 10.1016/j.cub.2019.11.054 Antonella Ruggiero 1 , Yuki Katou 2 , Katsuhiko Shirahige 2 , Martial Séveno 3 , Simonetta Piatti 1
Current Biology ( IF 9.2 ) Pub Date : 2020-01-09 , DOI: 10.1016/j.cub.2019.11.054 Antonella Ruggiero 1 , Yuki Katou 2 , Katsuhiko Shirahige 2 , Martial Séveno 3 , Simonetta Piatti 1
Affiliation
|
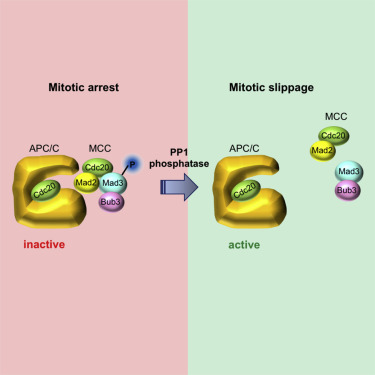
Accurate chromosome segregation requires bipolar attachment of kinetochores to spindle microtubules. A conserved surveillance mechanism, the spindle assembly checkpoint (SAC), responds to lack of kinetochore-microtubule connections and delays anaphase onset until all chromosomes are bipolarly attached [1]. SAC signaling fires at kinetochores and involves a soluble mitotic checkpoint complex (MCC) that inhibits the anaphase-promoting complex (APC) [2, 3]. The mitotic delay imposed by SAC, however, is not everlasting. If kinetochores fail to establish bipolar connections, cells can escape from the SAC-induced mitotic arrest through a process called mitotic slippage [4]. Mitotic slippage occurs in the presence of SAC signaling at kinetochores [5, 6], but whether and how MCC stability and APC inhibition are actively controlled during slippage is unknown. The PP1 phosphatase has emerged as a key factor in SAC silencing once all kinetochores are bipolarly attached [7, 8]. PP1 turns off SAC signaling through dephosphorylation of the SAC scaffold Knl1/Blinkin at kinetochores [9-11]. Here, we show that, in budding yeast, PP1 is also required for mitotic slippage. However, its involvement in this process is not linked to kinetochores but rather to MCC stability. We identify S268 of Mad3 as a critical target of PP1 in this process. Mad3 S268 dephosphorylation destabilizes the MCC without affecting the initial SAC-induced mitotic arrest. Conversely, it accelerates mitotic slippage and overcomes the slippage defect of PP1 mutants. Thus, slippage is not the mere consequence of incomplete APC inactivation that brings about mitotic exit, as originally proposed, but involves the exertive antagonism between kinases and phosphatases.
中文翻译:

磷酸酶PP1通过Mad3去磷酸化促进有丝分裂滑移。
准确的染色体分离需要动植物双极连接到纺锤体微管。一种保守的监视机制,即纺锤体装配检查点(SAC),对缺乏动粒体-微管连接作出反应,并延迟后期开始,直到所有染色体都双极性附着[1]。SAC信号在动植物处激发,并涉及可抑制后期促进复合物(APC)的可溶性有丝分裂检查点复合物(MCC)[2,3]。但是,SAC施加的有丝分裂延迟并非持久。如果动植物不能建立双极连接,则细胞可以通过称为有丝分裂滑移的过程从SAC诱导的有丝分裂停滞中逃脱[4]。有丝分裂中存在SAC信号时,发生有丝分裂滑移[5,6],但是在打滑过程中是否以及如何主动控制MCC稳定性和APC抑制作用尚不清楚。一旦所有动植物都双极性附着,PP1磷酸酶已成为SAC沉默的关键因素[7,8]。PP1通过在动植物的SAC支架Knl1 / Blinkin的去磷酸化来关闭SAC信号传导[9-11]。在这里,我们表明,在发芽的酵母中,PP1也是有丝分裂滑移所必需的。但是,它在此过程中的参与与动植物无关,而与MCC的稳定性有关。我们在此过程中将Mad3的S268确定为PP1的关键目标。Mad3 S268去磷酸化可稳定MCC,而不影响最初的SAC诱导的有丝分裂停滞。相反,它可加速有丝分裂滑移并克服PP1突变体的滑移缺陷。从而,
更新日期:2020-01-09
中文翻译:

磷酸酶PP1通过Mad3去磷酸化促进有丝分裂滑移。
准确的染色体分离需要动植物双极连接到纺锤体微管。一种保守的监视机制,即纺锤体装配检查点(SAC),对缺乏动粒体-微管连接作出反应,并延迟后期开始,直到所有染色体都双极性附着[1]。SAC信号在动植物处激发,并涉及可抑制后期促进复合物(APC)的可溶性有丝分裂检查点复合物(MCC)[2,3]。但是,SAC施加的有丝分裂延迟并非持久。如果动植物不能建立双极连接,则细胞可以通过称为有丝分裂滑移的过程从SAC诱导的有丝分裂停滞中逃脱[4]。有丝分裂中存在SAC信号时,发生有丝分裂滑移[5,6],但是在打滑过程中是否以及如何主动控制MCC稳定性和APC抑制作用尚不清楚。一旦所有动植物都双极性附着,PP1磷酸酶已成为SAC沉默的关键因素[7,8]。PP1通过在动植物的SAC支架Knl1 / Blinkin的去磷酸化来关闭SAC信号传导[9-11]。在这里,我们表明,在发芽的酵母中,PP1也是有丝分裂滑移所必需的。但是,它在此过程中的参与与动植物无关,而与MCC的稳定性有关。我们在此过程中将Mad3的S268确定为PP1的关键目标。Mad3 S268去磷酸化可稳定MCC,而不影响最初的SAC诱导的有丝分裂停滞。相反,它可加速有丝分裂滑移并克服PP1突变体的滑移缺陷。从而,



























 京公网安备 11010802027423号
京公网安备 11010802027423号