当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The influence of negatively charged silica nanoparticles on the surface properties of anionic surfactants: electrostatic repulsion or the effect of ionic strength?
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-01-09 , DOI: 10.1039/c9cp05475h Milad Eftekhari 1 , Karin Schwarzenberger 1 , Aliyar Javadi 2 , Kerstin Eckert 1
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2020-01-09 , DOI: 10.1039/c9cp05475h Milad Eftekhari 1 , Karin Schwarzenberger 1 , Aliyar Javadi 2 , Kerstin Eckert 1
Affiliation
|
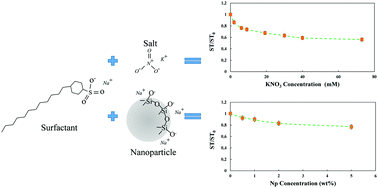
The presence of negatively charged nanoparticles affects the surface activity of anionic surfactants in an aqueous phase. Recent studies suggest that electrostatic repulsive forces play an important role in increasing the surface activity of surfactants. However, the addition of nanoparticles also increases the ionic strength of the system, which has a significant impact on the surfactant's properties, e.g. its critical micelle concentration (CMC). To investigate how and to what extent electrostatic forces and ionic strength influence the behavior of ionic surfactants, the surface tension and elasticity of different solutions were measured using drop profile tensiometry as a function of the surfactant (SDBS), nanoparticle (silica) and salt (KNO3) concentration. It is observed that the surface activity of the surfactants is mainly influenced by the change in the system's ionic strength due to the presence of nanoparticles. Several characteristic parameters including the equivalent concentration of the surfactant, the CMC and the apparent partial molar area of the adsorbed surfactant are theoretically calculated and further employed to validate experimental observations. Both the nanoparticles and electrolyte decrease the CMC, while the equivalent concentration of the surfactant remains nearly constant. This paper presents a criterion to estimate the possible influence of such forces for nanoparticles of different sizes and mass fractions.
中文翻译:

带负电的二氧化硅纳米颗粒对阴离子表面活性剂表面性能的影响:静电排斥还是离子强度的影响?
带负电的纳米粒子的存在会影响阴离子表面活性剂在水相中的表面活性。最近的研究表明静电排斥力在增加表面活性剂的表面活性中起重要作用。然而,纳米颗粒的添加也增加了系统的离子强度,这对表面活性剂的性质(例如其临界胶束浓度(CMC))具有重大影响。为了研究静电力和离子强度如何以及在多大程度上影响离子表面活性剂的行为,使用液滴轮廓张力法测量表面活性剂(SDBS),纳米颗粒(二氧化硅)和盐的函数来测量不同溶液的表面张力和弹性( KNO3)浓度。观察到,由于存在纳米颗粒,表面活性剂的表面活性主要受体系离子强度的变化影响。理论上计算了几个特征参数,包括表面活性剂的当量浓度,CMC和吸附的表面活性剂的表观部分摩尔面积,并进一步用于验证实验观察结果。纳米颗粒和电解质都降低了CMC,而表面活性剂的当量浓度几乎保持恒定。本文提出了一个标准,以估计这种力对不同尺寸和质量分数的纳米颗粒的可能影响。理论上计算了几个特征参数,包括表面活性剂的当量浓度,CMC和吸附的表面活性剂的表观部分摩尔面积,并进一步用于验证实验观察结果。纳米颗粒和电解质都降低了CMC,而表面活性剂的当量浓度几乎保持恒定。本文提出了一个标准,以估计这种力对不同尺寸和质量分数的纳米颗粒的可能影响。理论上计算了几个特征参数,包括表面活性剂的当量浓度,CMC和吸附的表面活性剂的表观部分摩尔面积,并进一步用于验证实验观察结果。纳米颗粒和电解质都降低了CMC,而表面活性剂的当量浓度几乎保持恒定。本文提出了一个标准,以估计这种力对不同尺寸和质量分数的纳米粒子的可能影响。
更新日期:2020-01-15
中文翻译:

带负电的二氧化硅纳米颗粒对阴离子表面活性剂表面性能的影响:静电排斥还是离子强度的影响?
带负电的纳米粒子的存在会影响阴离子表面活性剂在水相中的表面活性。最近的研究表明静电排斥力在增加表面活性剂的表面活性中起重要作用。然而,纳米颗粒的添加也增加了系统的离子强度,这对表面活性剂的性质(例如其临界胶束浓度(CMC))具有重大影响。为了研究静电力和离子强度如何以及在多大程度上影响离子表面活性剂的行为,使用液滴轮廓张力法测量表面活性剂(SDBS),纳米颗粒(二氧化硅)和盐的函数来测量不同溶液的表面张力和弹性( KNO3)浓度。观察到,由于存在纳米颗粒,表面活性剂的表面活性主要受体系离子强度的变化影响。理论上计算了几个特征参数,包括表面活性剂的当量浓度,CMC和吸附的表面活性剂的表观部分摩尔面积,并进一步用于验证实验观察结果。纳米颗粒和电解质都降低了CMC,而表面活性剂的当量浓度几乎保持恒定。本文提出了一个标准,以估计这种力对不同尺寸和质量分数的纳米颗粒的可能影响。理论上计算了几个特征参数,包括表面活性剂的当量浓度,CMC和吸附的表面活性剂的表观部分摩尔面积,并进一步用于验证实验观察结果。纳米颗粒和电解质都降低了CMC,而表面活性剂的当量浓度几乎保持恒定。本文提出了一个标准,以估计这种力对不同尺寸和质量分数的纳米颗粒的可能影响。理论上计算了几个特征参数,包括表面活性剂的当量浓度,CMC和吸附的表面活性剂的表观部分摩尔面积,并进一步用于验证实验观察结果。纳米颗粒和电解质都降低了CMC,而表面活性剂的当量浓度几乎保持恒定。本文提出了一个标准,以估计这种力对不同尺寸和质量分数的纳米粒子的可能影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号