当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unusual Reactivity of a Thiazole-Based Mn Tricarbonyl Complex for CO2 Activation
Organometallics ( IF 2.8 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.organomet.9b00727 Kundan K. Singh 1 , Maxime A. Siegler 1 , V. Sara Thoi 1
Organometallics ( IF 2.8 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.organomet.9b00727 Kundan K. Singh 1 , Maxime A. Siegler 1 , V. Sara Thoi 1
Affiliation
|
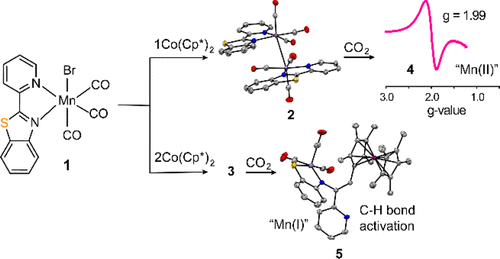
Manganese tricarbonyl complexes have attracted considerable attention as competent electrocatalysts for CO2 reduction owing to their unique chemistry and the natural abundance of Mn. While the analogous Re complexes are well-known to activate CO2 under anhydrous conditions, most reported Mn tricarbonyl catalysts require exogenous protons. In an effort to understand the reason for the different CO2 reduction mechanisms, we investigate the reactivity of Mn(pbt)(CO)3Br, (1, where pbt = 2-(2-pyridyl)-benzothiazole) with CO2. Our combined electrochemical and spectroscopic data suggest that 1 can undergo a CO2 conversion to CO similar to Re complexes with and without the presence of a proton source but only at potentials significantly more negative than the second reduction. Furthermore, the one- and two-electron reduced species are chemically generated and characterized by NMR, UV–vis, and IR spectroscopy. By exploring sequential chemical reduction of 1, we uncover unusual reactivity of the reduced dimeric Mn–Mn species as well as the isolation of a species capable of C–H activation.
中文翻译:

噻唑基锰三羰基锰配合物对CO 2活化的异常反应性
锰三羰基配合物由于其独特的化学性质和天然的Mn富集作为可还原CO 2的有效电催化剂吸引了相当多的关注。虽然众所周知类似的Re络合物在无水条件下活化CO 2,但大多数报道的Mn三羰基Mn催化剂需要外源质子。为了了解不同的CO 2还原机理的原因,我们研究了Mn(pbt)(CO)3 Br,(1,其中pbt = 2-(2-吡啶基)-苯并噻唑)与CO 2的反应性。我们的电化学和光谱综合数据表明1可以经历CO 2在有或没有质子源的情况下,转化为类似于Re络合物的CO,但其电势远比第二次还原更负。此外,一电子和二电子还原的物种是化学生成的,并通过NMR,UV-vis和IR光谱进行表征。通过探索连续的化学还原1,我们发现了还原的二聚体Mn-Mn物种的异常反应性以及能够进行CH活化的物种的分离。
更新日期:2020-01-08
中文翻译:

噻唑基锰三羰基锰配合物对CO 2活化的异常反应性
锰三羰基配合物由于其独特的化学性质和天然的Mn富集作为可还原CO 2的有效电催化剂吸引了相当多的关注。虽然众所周知类似的Re络合物在无水条件下活化CO 2,但大多数报道的Mn三羰基Mn催化剂需要外源质子。为了了解不同的CO 2还原机理的原因,我们研究了Mn(pbt)(CO)3 Br,(1,其中pbt = 2-(2-吡啶基)-苯并噻唑)与CO 2的反应性。我们的电化学和光谱综合数据表明1可以经历CO 2在有或没有质子源的情况下,转化为类似于Re络合物的CO,但其电势远比第二次还原更负。此外,一电子和二电子还原的物种是化学生成的,并通过NMR,UV-vis和IR光谱进行表征。通过探索连续的化学还原1,我们发现了还原的二聚体Mn-Mn物种的异常反应性以及能够进行CH活化的物种的分离。

























 京公网安备 11010802027423号
京公网安备 11010802027423号