当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Fluxional Nature of the Hydrated Electron: Energy and Entropy Contributions to Aqueous Electron Free Energies.
Journal of Chemical Theory and Computation ( IF 5.5 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.jctc.9b00496 William J Glover 1, 2, 3 , Benjamin J Schwartz 4
Journal of Chemical Theory and Computation ( IF 5.5 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.jctc.9b00496 William J Glover 1, 2, 3 , Benjamin J Schwartz 4
Affiliation
|
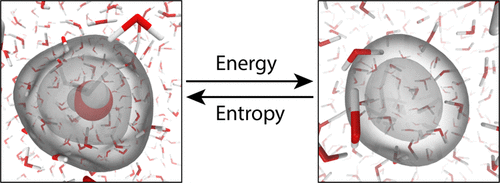
There has been a great deal of recent controversy over the structure of the hydrated electron and whether it occupies a cavity or contains a significant number of interior waters (noncavity). The questions we address in this work are, from a free energy perspective, how different are these proposed structures? Do the different structures all lie along a single continuum, or are there significant differences (i.e., free energy barriers) between them? To address these questions, we have performed a series of one-electron calculations using umbrella sampling with quantum biased molecular dynamics along a coordinate that directly reflects the number of water molecules in the hydrated electron's interior. We verify that a standard cavity model of the hydrated electron behaves essentially as a hard sphere: the model is dominated by repulsion at short range such that water is expelled from a local volume around the electron, leading to a water solvation shell like that of a pseudohalide ion. The repulsion is much larger than thermal energies near room temperature, explaining why such models exhibit properties with little temperature dependence. On the other hand, our calculations reveal that a noncavity model is highly fluxional, meaning that thermal motions cause the number of interior waters to fluctuate from effectively zero (i.e., a cavity-type electron) to potentially above the bulk water density. The energetic contributions in the noncavity model are still repulsive in the sense that they favor cavity formation, so the fluctuations in structure are driven largely by entropy: the entropic cost for expelling water from a region of space is large enough that some water is still driven into the electron's interior. As the temperature is lowered and entropy becomes less important, the noncavity electron's structure is predicted to become more cavity-like, consistent with the observed temperature dependence of the hydrated electron's properties. Thus, we argue that although the specific noncavity model we study overestimates the preponderance of fluctuations involving interior water molecules, with appropriate refinements to correctly capture the true average number of interior waters and molar solvation volume, a fluxional model likely makes the most sense for understanding the various experimental properties of the hydrated electron.
中文翻译:

水合电子的通量性质:水电子自由能的能量和熵贡献。
关于水合电子的结构及其是否占据空腔或是否包含大量内部水(无空腔),最近有很多争议。从自由能源的角度来看,我们在这项工作中要解决的问题是这些拟议的结构有何不同?不同的结构是否全部都位于一个连续体上,或者它们之间是否存在显着差异(即自由能垒)?为了解决这些问题,我们使用伞式采样进行了一系列单电子计算,该采样采用沿轴向直接反映水合电子内部水分子数量的量子偏置分子动力学。我们验证了水合电子的标准腔模型本质上表现为硬球:该模型主要受短距离排斥作用的影响,因此水会从电子周围的局部体积中排出,从而形成水化壳,就像拟卤化物离子一样。排斥力比室温附近的热能大得多,这解释了为什么这种模型表现出几乎不依赖温度的特性。另一方面,我们的计算表明,非空穴模型具有很高的通量,这意味着热运动会导致内部水的数量从有效零(即腔型电子)波动到可能高于总体水密度。从非空洞模型的能量贡献看似有利于形成空腔的意义上,它们仍然是排斥性的,因此结构的波动主要由熵驱动:从空间区域排出水的熵成本足够大,以至于仍有一些水被驱入电子内部。随着温度降低和熵变得不那么重要,预计非空穴电子的结构将变得更像腔,这与水合电子性质的温度依赖性有关。因此,我们认为,尽管我们研究的特定非空穴模型高估了涉及内部水分子的波动,但通过适当地细化以正确捕获内部水的真实平均数量和摩尔溶剂化量,通量模型可能最能理解水合电子的各种实验性质。随着温度的降低和熵的重要性降低,预计非空穴电子的结构将变得更像腔,这与水合电子性质的温度依赖性有关。因此,我们认为,尽管我们研究的特定非空穴模型高估了涉及内部水分子的波动,但通过适当地细化以正确捕获内部水的真实平均数量和摩尔溶剂化量,通量模型可能最能理解水合电子的各种实验性质。随着温度的降低和熵的重要性降低,预计非空穴电子的结构将变得更像腔,这与水合电子性质的温度依赖性有关。因此,我们认为,尽管我们研究的特定非空穴模型高估了涉及内部水分子的波动,但通过适当地细化以正确捕获内部水的真实平均数量和摩尔溶剂化量,通量模型可能最能理解水合电子的各种实验性质。与观察到的水合电子性质的温度依赖性一致。因此,我们认为,尽管我们研究的特定非空穴模型高估了涉及内部水分子的波动,但通过适当地细化以正确捕获内部水的真实平均数量和摩尔溶剂化量,通量模型可能最有助于理解水合电子的各种实验性质。与观察到的水合电子性质的温度依赖性一致。因此,我们认为,尽管我们研究的特定非空穴模型高估了涉及内部水分子的波动,但通过适当地细化以正确捕获内部水的真实平均数量和摩尔溶剂化量,通量模型可能最有助于理解水合电子的各种实验性质。
更新日期:2020-01-23
中文翻译:

水合电子的通量性质:水电子自由能的能量和熵贡献。
关于水合电子的结构及其是否占据空腔或是否包含大量内部水(无空腔),最近有很多争议。从自由能源的角度来看,我们在这项工作中要解决的问题是这些拟议的结构有何不同?不同的结构是否全部都位于一个连续体上,或者它们之间是否存在显着差异(即自由能垒)?为了解决这些问题,我们使用伞式采样进行了一系列单电子计算,该采样采用沿轴向直接反映水合电子内部水分子数量的量子偏置分子动力学。我们验证了水合电子的标准腔模型本质上表现为硬球:该模型主要受短距离排斥作用的影响,因此水会从电子周围的局部体积中排出,从而形成水化壳,就像拟卤化物离子一样。排斥力比室温附近的热能大得多,这解释了为什么这种模型表现出几乎不依赖温度的特性。另一方面,我们的计算表明,非空穴模型具有很高的通量,这意味着热运动会导致内部水的数量从有效零(即腔型电子)波动到可能高于总体水密度。从非空洞模型的能量贡献看似有利于形成空腔的意义上,它们仍然是排斥性的,因此结构的波动主要由熵驱动:从空间区域排出水的熵成本足够大,以至于仍有一些水被驱入电子内部。随着温度降低和熵变得不那么重要,预计非空穴电子的结构将变得更像腔,这与水合电子性质的温度依赖性有关。因此,我们认为,尽管我们研究的特定非空穴模型高估了涉及内部水分子的波动,但通过适当地细化以正确捕获内部水的真实平均数量和摩尔溶剂化量,通量模型可能最能理解水合电子的各种实验性质。随着温度的降低和熵的重要性降低,预计非空穴电子的结构将变得更像腔,这与水合电子性质的温度依赖性有关。因此,我们认为,尽管我们研究的特定非空穴模型高估了涉及内部水分子的波动,但通过适当地细化以正确捕获内部水的真实平均数量和摩尔溶剂化量,通量模型可能最能理解水合电子的各种实验性质。随着温度的降低和熵的重要性降低,预计非空穴电子的结构将变得更像腔,这与水合电子性质的温度依赖性有关。因此,我们认为,尽管我们研究的特定非空穴模型高估了涉及内部水分子的波动,但通过适当地细化以正确捕获内部水的真实平均数量和摩尔溶剂化量,通量模型可能最能理解水合电子的各种实验性质。与观察到的水合电子性质的温度依赖性一致。因此,我们认为,尽管我们研究的特定非空穴模型高估了涉及内部水分子的波动,但通过适当地细化以正确捕获内部水的真实平均数量和摩尔溶剂化量,通量模型可能最有助于理解水合电子的各种实验性质。与观察到的水合电子性质的温度依赖性一致。因此,我们认为,尽管我们研究的特定非空穴模型高估了涉及内部水分子的波动,但通过适当地细化以正确捕获内部水的真实平均数量和摩尔溶剂化量,通量模型可能最有助于理解水合电子的各种实验性质。


























 京公网安备 11010802027423号
京公网安备 11010802027423号