当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cobalt-Based MOF-on-MOF Two-Dimensional Heterojunction Nanostructures for Enhanced Oxygen Evolution Reaction Electrocatalytic Activity.
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.inorgchem.9b03011 Qingqing Zha 1 , Feifei Yuan 1 , Guoxu Qin 1 , Yonghong Ni 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-01-09 , DOI: 10.1021/acs.inorgchem.9b03011 Qingqing Zha 1 , Feifei Yuan 1 , Guoxu Qin 1 , Yonghong Ni 1
Affiliation
|
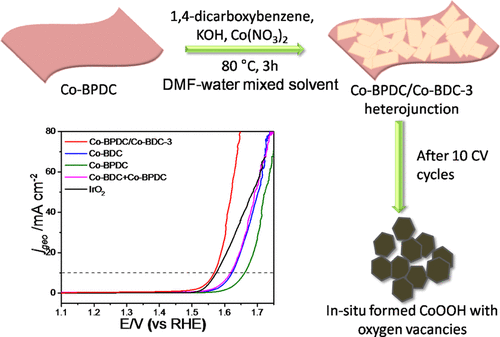
Two-dimensional (2D) Co-based MOF-on-MOF heterojunction nanostructures with improved electrocatalytic activity were successfully constructed via a mild two-step solution route, employing Co2+ ions as the center atoms, and 1,4-benzenedicarboxylate (BDC) and 4,4'-biphenyldicarboxylate (BPDC) as ligands. The as-obtained heterojunction nanostructures were characterized by field-emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, Brunauer-Emmett-Teller (BET) surface area analysis, thermogravimetric analysis (TGA), and X-ray photoelectron spectroscopy (XPS) technologies. Electrochemical measurements showed that as-prepared Co-BPDC/Co-BDC heterojunction nanostructures presented markedly enhanced OER electrocatalytic activity, compared with single Co-BPDC, Co-BDC, and/or their physical mixture. Also, the Co-BPDC/Co-BDC-3 heterojunction prepared after treatment for 3 h exhibited the strongest catalytic activity. To reach the current density jgeo = 10 mA cm-2, the Co-BPDC/Co-BDC-3 heterojunction-modified glassy carbon electrode required an overpotential of 335 mV in 1 M KOH, which was reduced by 57 and 93 mV, compared to the electrodes modified by Co-BDC and Co-BPDC, respectively. Simultaneously, the heterojunction catalyst also displayed better long-term stability. The improvement of the above performances should be attributed to the increased structure stability, BET surface area, ECSA, and electron transfer ability of the heterojunction.
中文翻译:

钴基MOF-on-MOF二维异质结纳米结构,用于增强氧释放反应的电催化活性。
通过以Co2 +离子为中心原子,1,4-苯二甲酸(BDC)和碳原子数为2的温和两步溶液法成功构建了具有改进的电催化活性的二维(2D)Co基MOF-on-MOF异质结纳米结构。 4,4'-联苯二羧酸酯(BPDC)作为配体。通过场发射扫描电子显微镜(FESEM),X射线衍射(XRD),傅里叶变换红外(FTIR)光谱,Brunauer-Emmett-Teller(BET)表面积分析,热重分析( TGA)和X射线光电子能谱(XPS)技术。电化学测量表明,与单一的Co-BPDC,Co-BDC相比,制备的Co-BPDC / Co-BDC异质结纳米结构具有显着增强的OER电催化活性。和/或其物理混合物。同样,处理3小时后制备的Co-BPDC / Co-BDC-3异质结表现出最强的催化活性。为了达到电流密度jgeo = 10 mA cm-2,Co-BPDC / Co-BDC-3异质结修饰的玻碳电极在1 M KOH中需要335 mV的过电势,与之相比,该电势降低了57和93 mV分别由Co-BDC和Co-BPDC修饰的电极。同时,异质结催化剂也表现出更好的长期稳定性。上述性能的改善应归因于增加的结构稳定性,BET表面积,ECSA和异质结的电子转移能力。为了达到电流密度jgeo = 10 mA cm-2,Co-BPDC / Co-BDC-3异质结修饰的玻碳电极在1 M KOH中需要335 mV的过电势,与之相比,该电势降低了57和93 mV分别由Co-BDC和Co-BPDC修饰的电极。同时,异质结催化剂也表现出更好的长期稳定性。上述性能的改善应归因于增加的结构稳定性,BET表面积,ECSA和异质结的电子转移能力。为了达到电流密度jgeo = 10 mA cm-2,Co-BPDC / Co-BDC-3异质结修饰的玻碳电极在1 M KOH中需要335 mV的过电势,与之相比,该电势降低了57和93 mV分别由Co-BDC和Co-BPDC修饰的电极。同时,异质结催化剂也表现出更好的长期稳定性。上述性能的改善应归因于增加的结构稳定性,BET表面积,ECSA和异质结的电子转移能力。
更新日期:2020-01-09
中文翻译:

钴基MOF-on-MOF二维异质结纳米结构,用于增强氧释放反应的电催化活性。
通过以Co2 +离子为中心原子,1,4-苯二甲酸(BDC)和碳原子数为2的温和两步溶液法成功构建了具有改进的电催化活性的二维(2D)Co基MOF-on-MOF异质结纳米结构。 4,4'-联苯二羧酸酯(BPDC)作为配体。通过场发射扫描电子显微镜(FESEM),X射线衍射(XRD),傅里叶变换红外(FTIR)光谱,Brunauer-Emmett-Teller(BET)表面积分析,热重分析( TGA)和X射线光电子能谱(XPS)技术。电化学测量表明,与单一的Co-BPDC,Co-BDC相比,制备的Co-BPDC / Co-BDC异质结纳米结构具有显着增强的OER电催化活性。和/或其物理混合物。同样,处理3小时后制备的Co-BPDC / Co-BDC-3异质结表现出最强的催化活性。为了达到电流密度jgeo = 10 mA cm-2,Co-BPDC / Co-BDC-3异质结修饰的玻碳电极在1 M KOH中需要335 mV的过电势,与之相比,该电势降低了57和93 mV分别由Co-BDC和Co-BPDC修饰的电极。同时,异质结催化剂也表现出更好的长期稳定性。上述性能的改善应归因于增加的结构稳定性,BET表面积,ECSA和异质结的电子转移能力。为了达到电流密度jgeo = 10 mA cm-2,Co-BPDC / Co-BDC-3异质结修饰的玻碳电极在1 M KOH中需要335 mV的过电势,与之相比,该电势降低了57和93 mV分别由Co-BDC和Co-BPDC修饰的电极。同时,异质结催化剂也表现出更好的长期稳定性。上述性能的改善应归因于增加的结构稳定性,BET表面积,ECSA和异质结的电子转移能力。为了达到电流密度jgeo = 10 mA cm-2,Co-BPDC / Co-BDC-3异质结修饰的玻碳电极在1 M KOH中需要335 mV的过电势,与之相比,该电势降低了57和93 mV分别由Co-BDC和Co-BPDC修饰的电极。同时,异质结催化剂也表现出更好的长期稳定性。上述性能的改善应归因于增加的结构稳定性,BET表面积,ECSA和异质结的电子转移能力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号