当前位置:
X-MOL 学术
›
Gene Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Transposon-mediated generation of CAR-T cells shows efficient anti B-cell leukemia response after ex vivo expansion.
Gene Therapy ( IF 5.1 ) Pub Date : 2020-01-09 , DOI: 10.1038/s41434-020-0121-4 Leonardo Chicaybam 1, 2 , Luiza Abdo 1 , Mariana Viegas 1 , Luisa Vieira Codeço Marques 1 , Priscila de Sousa 1 , Leonardo Ribeiro Batista-Silva 1 , Viviane Alves-Monteiro 1 , Simone Bonecker 3 , Bárbara Monte-Mór 3 , Martín Hernán Bonamino 1, 2
Gene Therapy ( IF 5.1 ) Pub Date : 2020-01-09 , DOI: 10.1038/s41434-020-0121-4 Leonardo Chicaybam 1, 2 , Luiza Abdo 1 , Mariana Viegas 1 , Luisa Vieira Codeço Marques 1 , Priscila de Sousa 1 , Leonardo Ribeiro Batista-Silva 1 , Viviane Alves-Monteiro 1 , Simone Bonecker 3 , Bárbara Monte-Mór 3 , Martín Hernán Bonamino 1, 2
Affiliation
|
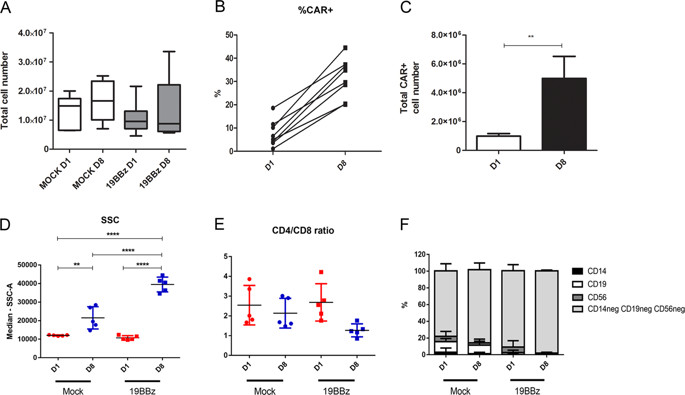
CAR-T-cell therapy has shown considerable advance in recent years, being approved by regulatory agencies in US, Europe, and Japan for the treatment of refractory patients with CD19+ B-cell leukemia or diffuse large B-cell lymphoma. Current methods for CAR-T-cell production use viral vectors for T-cell genetic modification and can take up to 15 days to generate the infusion product. The development of simple and less costly manufacturing protocols is needed in order to meet the increasing demand for this therapy. In this present work, we generated 19BBz CAR-T cells in 8 days using a protocol based on the non-viral transposon-based vector Sleeping Beauty. The expanded cells display mostly a central memory phenotype, expressing higher levels of inhibitory receptors when compared with mock cells. In addition, CAR-T cells were cytotoxic against CD19+ leukemia cells in vitro and improved overall survival rates of mice xenografted with human RS4;11 or Nalm-6 B-cell leukemias. Infused CAR-T cells persisted for up to 28 days, showing that they are capable of long-term persistence and antitumor response. Altogether, these results demonstrate the effectiveness of our protocol and pave the way for a broader application of CAR-T-cell therapy.
中文翻译:

转座子介导的CAR-T细胞生成在离体扩增后显示出有效的抗B细胞白血病反应。
近年来,CAR-T细胞疗法已显示出相当大的进步,已获得美国,欧洲和日本监管机构的批准,可用于治疗难治性CD19 + B细胞白血病或弥漫性大B细胞淋巴瘤患者。目前用于CAR-T细胞生产的方法使用病毒载体进行T细胞遗传修饰,最多可能需要15天才能产生输注产物。为了满足对该疗法日益增长的需求,需要开发简单且成本较低的制造方案。在本研究中,我们使用基于非病毒转座子的载体Sleeping Beauty的方案在8天之内生成了19BBz CAR-T细胞。与模拟细胞相比,扩增的细胞主要表现出中央记忆表型,表达更高水平的抑制受体。此外,CAR-T细胞在体外对CD19 +白血病细胞具有细胞毒性,并提高了异种移植人RS4; 11或Nalm-6 B细胞白血病小鼠的总生存率。注入的CAR-T细胞可持续长达28天,表明它们具有长期的持久性和抗肿瘤反应能力。总而言之,这些结果证明了我们方案的有效性,为CAR-T细胞疗法的更广泛应用铺平了道路。
更新日期:2020-01-09
中文翻译:

转座子介导的CAR-T细胞生成在离体扩增后显示出有效的抗B细胞白血病反应。
近年来,CAR-T细胞疗法已显示出相当大的进步,已获得美国,欧洲和日本监管机构的批准,可用于治疗难治性CD19 + B细胞白血病或弥漫性大B细胞淋巴瘤患者。目前用于CAR-T细胞生产的方法使用病毒载体进行T细胞遗传修饰,最多可能需要15天才能产生输注产物。为了满足对该疗法日益增长的需求,需要开发简单且成本较低的制造方案。在本研究中,我们使用基于非病毒转座子的载体Sleeping Beauty的方案在8天之内生成了19BBz CAR-T细胞。与模拟细胞相比,扩增的细胞主要表现出中央记忆表型,表达更高水平的抑制受体。此外,CAR-T细胞在体外对CD19 +白血病细胞具有细胞毒性,并提高了异种移植人RS4; 11或Nalm-6 B细胞白血病小鼠的总生存率。注入的CAR-T细胞可持续长达28天,表明它们具有长期的持久性和抗肿瘤反应能力。总而言之,这些结果证明了我们方案的有效性,为CAR-T细胞疗法的更广泛应用铺平了道路。


























 京公网安备 11010802027423号
京公网安备 11010802027423号